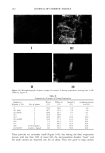
JEWELRY POWDERS AND FAR-INFRARED RAYS 177 jade powder, T-cream contained 1% tourmaline powder, and the control cream con- tained water and oils only. SCANNING ELECTRON MICROSCOPY (SEM) The powders underwent a gold-palladium coating. The observations were realized with a Hitachi S4300 (Hitachi, Japan) scanning electron microscope. FAR-INFRARED RAY ANALYSIS Sample preparation. Each of the jewelry powders was formulated in a cosmetic O/W emulsion. The contents of the jewelry powder were from 0.1% to 7% (w/w). After formulation, they were completely dried in a vacuum oven for 48 hours at 60 ø. Far-infrared ray analysis. Emissivity and emission energies were observed with an IR spectrometer (M2410-C, Midac Co., USA), and the observed wave ranges were 4 to 20 micrometers. THERMOGAPHIC SKIN TEMPERATURE ANALYSIS Preparation of O/W emulsiom. The control cream was formulated with oil, surfactant, and water only, and contained no mineral compounds. Each of the sample creams was formulated with the jade powder and the tourmaline powder. The J-cream was the sample cream containing the jade powder, and the T-cream was that containing the tourmaline powder. The contents of the jewelry powder were 0.25%, 1.0%, 3.0%, and 7.0% (w/w). Subjects and thermography. The subjects were five healthy females and five healthy males aged 26 to 35 years. Each subject washed his or her face using a liquid face-washing agent (Pacific Co.) and water. The subjects were thermographed (Thermovision 900, AGEMA, Sweden) in a room with a constant temperature of 25 ø and a constant relative humidity of 40% to 50%. Ten minutes after washing his or her face, each subject was seated on a chair. Five minutes after seating, the first thermograph was taken. Each subject treated his or her face with 0.5 g of control cream (right side) and 0.5 g of sample cream (left side). Immediately after application of the creams, the second thermograph was taken. The thermographs were all taken according to a pre-fixed schedule. RESULTS AND DISCUSSION The chemical composition of the tourmaline was (Na,Ca)(Mg,Li,A1,Fe2+)3AI6B3Si 6 (OH)4. It had a fibrous structure and a hardness of 7 to 7.5, a specific gravity of 3.0 to 3.3, and a refractive index of 1.625 to 1.655. Tourmaline usually occurs as heavily striated, elongated, prismatic crystals, and less commonly as short, stubby, prismatic crystals. All tourmaline crystals have a rounded, triangular cross section. Tourmaline seldom occurs in tabular crystals, but it also occurs in columnar, radiating, and stalactitic forms, as well as in dense groups of tiny, elongated needles and in black, compact masses.
178 JOURNAL OF COSMETIC SCIENCE Figure 1. SEM observation of tourmaline powders (x 1000). The crystal structure used was a mixture of a round-type crystal and a plane-type crystal, and had a mean diameter of 10 micrometers. The crystal structure used was a mixture of a round-type crystal and a plane-type crystal, and it had a mean diameter of 10 micrometers. The SEM image is shown in Figure 1. The chemical composition of the jade (nephrite) was Ca(Mg, Fe)5SisO22(OH) 2, and had a fibrous and planar structure. The hardness was 6 to 6.5, the specific gravity was 3.0, Figure 2. SEM observation of jade powders (x 2000). The crystal structure was a mixture of a needle-type crystal and a plane-type crystal, and had a mean diameter of 10 micrometers.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)