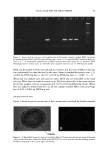
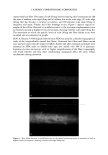
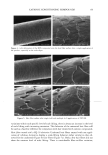
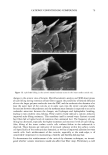
2002 ANNUAL SCIENTIFIC MEETING 103 presence of an orthorhombic lateral packing has been established. Recently the lateral packing has also been studied by electron diffraction using sequential strips of SC (2). With this approach we have established that throughout the SC the lipids form an orthorhombic lattice with the exception of the most superficial layers in which occasionally hexagonal packing has been observed. CHO]L:CER:FFA mixtures: In mixtures prepared from CHOL and CER (isolated from human SC), t•vo lamellar phases are formed with periodicities of 5.4 and 12.8 nm, respectively (3). This presence of the lamellar phases is insensitive to the CHOL:CER. In the equimolar CHOL:CER:FFA mixture the periodicity of the lamellar phases increases to 5.5 and 13.0 nm, respectively, mimicking even closer the lamellar phase behaviour in intact SC. Lateral packing: In the absence of FFA, a diffraction pattern indicating a hexagonal packing is observed. Addition of FFA induces a transition from a hexagonal to an orthorhombic packing, demonstrating a crucial role of FFA for the formation of a competent barrier (3). CHOL:CER:FFA mixtures prepared in the absence of CERI: When CER1 is absent only a very small population of lipids forms the 12.8 nm lamellar phase. Molecular organisation in the repeating unit of the 12-13 nm lamellar phase: The presence of a broad- narrow-broad sequence of the electron lucent bands of the lamellae in the RuO4-fixed SC (4) and an electron density distribution profile in the repeating unit of the 12.8 nm lamellar phase demonstrate a broad-narrow-broad sequence of the hydrocarbon regions. Based on this and on the key role CER1 plays in the formation of the 12-13 nm lamellar phase we proposed a model for molecular organization of this phase presented in figure 2. In this model the lipids are organised in 3 layers: a narrow layer that is located in the center with a broad layer on both sides. CER 1 links this tri-layer unit together and consequently dictates the broad-narrow-broad sequence in the tri-layer unit (4). It has been demonstrated that also in isolated model systems and in intact SC the orthorhombic phase coexists with the fluid phase. We propose that this liquid sublattice is located in domains in the central layer of the 12-13 nm phase. In this layer the unsaturated linoleate and cholesterol are present and that this fluid phase will acts as a diffusion pathway of substances and might facilitate the deformation of the lipid lamellae in the SC. This model is referred to as the "sandwich model". Conclusions:The lipids are organized in crystalline lamellae, but most probably a liquid phase is also present. The important role of CER1 in the formation of the 13.4 nm lamellar phase and the transition from a hexagonal to an orthorhombic phase induced by FFA have been observed in mixtures prepared with isolated SC lipids as well as in intact human SC. The in vitro findings can predict in vivo situation. SANDWICH MODEL 12-13 nm periodicity Figure 2 ]•$' "l'•l•i '•"•"•'.•/• 3,avers: A "sandwich model" is propos The Ir •' • • I• •b Crystaliineiattice ' '. i-•.• characteristics are: a liquid sublattice is in the • '• . . .-- ,Fluid phase central lipid layer of this phase and a gradual -'-- •...'..'_'. Crystaiiinelattice change in lipid mobility occurs due the I I presence of less mobile long saturated Gradual change in chain Linoleate CERt hydrocarbon chains. The central lipid layer is mobility Stacking of lamellae: aiternating fluid and crystalline sublattices not a continuous fluid phase ß . . •v.. _•.• - • •(Shear stress r ::: •:..i...• . -• :.: '•: -• Sandwich model: t : .. • _, _ _ . :7'/: •:•:!:::!: • ': : -• facilitates r "::: :•' ...... -• deformation 1 Bouwstra JA, Gooris GS, van der Spek JA and Bras W. J Invest Dermatol. 1991 96: 1006-1014. 2 Pilgram GSK, Engelsma-van Pelt AM, Bouwstra JA, Koerten HK. J Invest Dermatol 1999 133:403-409 3 Bouwstra JA, Gooris GS, Dubbelaar, FER, Ponce M, J. Invest. Dermatol. 2002 118: 606-616. 4 Madison KC, Swarzendruber DC, Wertz PW, Downing DT, J. Invest Dermatol 1987 88:714-718
104 JOURNAL OF COSMETIC SCIENCE CLINICAL EFFICACY OF 2% POLYPHENONE (GREEN TEA EXTRACT) IN A HYDROPHILIC GEL ON AGED AND DAMAGED FACIAL SKIN Tanweer A. Syed •, Ph.D., Wendy Hiu-Wai Wong 2 and Seyed A. Ahmad 3 •Department of Dermatology, University of California San Francisco 2BWT Group, Inc., 1032 Irving Street, #917, San Francisco, CA 94122 SDepartment of Chemistry, University of California, Berkeley, CA Introduction: Polypenolic Ëactions and the chemistry of -EGCg (Epigallocatechin gallate) or green tea extract molecules exhibit a variety of antioxidants that can regulate cell division, proliferation, platelet aggregation, and detoxification (1,2). Obviously, such properties can significantly contribute to impart clinically beneficial response to stimulate the dermis to form new collagen and elastin (the support fibers that prevent the skin from sagging and wrinkling) and also help in slowing or reversing the aging process. Additional, clinical data has also shown that green tea extract can also inhibit the release of catecholamines and the formation of SNARE complex (3,4). Objective: To evaluate the clinical efficacy and beneficial effects of 2% (-EGCg) green tea extract in a hydrophilic gel (1% hydroxyethylcellulose solution) on aged and damaged facial skin. Methods: Preselected, female panelist (n=60), aged 25-60 years showing visible signs of damaged facial skin observed by uneven skin texture and large pores were sequentially randomized into two parallel groups (active and placebo). Each subject was allocated an identical pre-coded tube (50-g) with instructions on how to topically apply the trial gel two times a day for 4 weeks. Clinical efficacy and beneficial effects were assessed by dermatological evaluations, such as, irritation, changes in skin firmness and elasticity and instrumental measurement of skin hydration. Photographic and optical techniques were used both at the baseline and on a weekly basis. Efficacy data regarding anti-aging and damaged facial skin was evaluated by both in vitro and in vivo tests. Inhibition in the release of catecholamines was ascertained by monitoring the neurotransmitters Adrenaline and Noradrenaline. Chromaffin cells (prepared from bovine adrenal glands by collagenase digestion and separated from erythrocytes) were incubated with adrenalin/noradrenaline and the trial gel. Both the release of catecholamines and the total cell count were determined by liquid scintillation. Evaluation of SNARE complex inhibition was also conducted on chromaffin cells. Skin topography analysis (in vivo) was performed by obtaining silicon imprints from around the eyes and the damaged facial skin of the subjects. Such imprints were obtained at 0 (baseline), 15 and after 30 days of two times a day regimen. Analysis of the imprints were performed by confocal laser scanning microscopy. The tolerability of the study gel was assessed at the scheduled visit by direct questioning of the subjects about study gel related subjective and objective adverse symptoms. For safety and toxicological evaluation, cytotoxicity tests on human epidermal keratinocytes and human dermal fibroblasts were also performed before the initiation of the trial. An informed consent was obtained from all the subjects. Study participants were requested not to use any emollient or topical application during the study period. The X • and Fisher's exact tests, with two-tailed figures were used for significant test results.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)