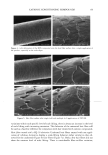
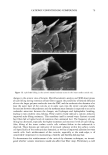
32 JOURNAL OF COSMETIC SCIENCE compartment. The solution in the donor compartment, referred to hereafter as the contacting solution, contained mixtures of SDS and PEO, each with about 0.5 pCi/ml of 14C-SDS. We verified that the concentration of radiolabeled SDS in the contacting solution did not change appreciably during the five-hour exposure to the skin. Diffusion into the skin took place for five hours, and subsequently the contacting solution was removed and the donor compartment was rinsed four times with 2 ml of PBS. A five-hour exposure was chosen because this was a sufficiently long time to allow significant SDS penetration into the skin, but a short enough time to prevent the saturation of the skin with SDS. The temperature of the diffusion cell was ambient, that is, 20 + IøC. The skin was then heat-stripped by placing it in a bath of water at 60øC for two minutes, and then peeling off the epidermis (SC and viable epidermis) that had been exposed to the contacting solution from the dermis. The exposed epidermis was then dried for two days in a fume hood and weighed. The dried epidermis was dissolved overnight in 1.5 ml of Soluene-350 (Packard, Meriden, CT). After the epidermis dis- solved, 10 ml of Hionic Fluor scintillation cocktail (Packard) was added to the Soluene- 350, and the concentration of radiolabeled SDS was determined using a Packard Tri- Carb 4350 scintillation counter (Packard). Knowing the concentration of SDS in the contacting solution, Csos, the radioactivity of the contacting solution, Crad, donor, the dry weight of the epidermis, m, and the radioactivity of the epidermis, Cr•,skin , it was possible to determine the concentration of SDS in the dried epidermis, CSOS, skin , using the following equation: C r•d, •kin ' CSDS (1) C SDS, skin z Grad, donor ' m DYNAMIC LIGHT SCATTERING The SDS and SDS+PEO solutions were prepared in Millipore-filtered water with 0.1 M NaCI. After mixing, the solutions were filtered through a 0.02 pm Anotop 10 syringe filter (Whatman International, Maidstone, England) directly into a cylindrical- scattering cell to remove any dust from the solution, and then sealed until use. Dynamic light scattering (DLS) (33) was performed at 25øC and a 90 ø scattering angle on a Brookhaven BI-200SM system (Brookhaven, Holtsville, NY) using a 2017 Stabilite argon-ion laser (Spectra Physics) at 488 nm. The autocorrelation function was analyzed using the CONTIN program provided by the BIC dynamic light scattering software -- (Brookhaven, Holtsville, NY), which determines the effective hydrodynamic radius, using the Stokes-Einstein relation (34): kBT -- Rh - 6q. rxl• (2) where kB is the Boltzmann constant, T is the absolute temperature, x I is the viscosity of -- the salt solution, and D is the mean diffusion coefficient of the scattering species. In order to measure the size of the micelies, including free SDS micelles and PEO-bound SDS micelies, and eliminate the effects of interparticle interactions, the effective hydro- dynamic radii were determined at several different SDS concentrations, and the average effective hydrodynamic radii were extrapolated to a zero micelie concentration (33,35-
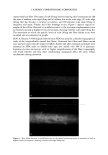
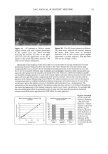
EPIDERMAL PENETRATION OF SDS MICELLES 33 38). We added 100 mM of NaCl to screen the intermicellar electrostatic interactions in the DLS measurements (33,36-38). In the presence of PEO, the concentration of PEO was adjusted such that only PEO- bound SDS micelies were predicted to exist (39). This was done to minimize the scattering from either the free SDS micelies or the free PEO molecules, both of which have R3 values that are similar to that of the PEO-bound SDS micelies. The concen- trations of SDS and PEO utilized in the DLS studies are listed in Table I. DETERMINATION OF THE AQUEOUS PORE RADIUS IN THE STRATUM CORNEUM Although we did not measure the aqueous pore radii ourselves, we believe that a brief discussion of the measurement method is in order. Using hindered-transport theories (40), several authors have measured the average aqueous pore radius in the SC (12,41- 44). In this method, the transdermal permeabilities of two types of polar molecules, or the transdermal permeability of a polar molecule and the transdermal conductivity of an ion, are measured. The permeants are either both radiolabeled molecules (12,43) or a radiolabeled molecule and an ion (42,44), which have different hydrodynamic radii. The effect of the skin pore radius on the diffusion of the permeant depends on the ratio of the permeant hydrodynamic radius to the pore radius, )•, with an increase in the hindrance to the diffusion of the permeant across the skin as )• approaches 1. Accordingly, by comparing the hindrance to diffusion across the skin of the two molecules, one can determine the effective aqueous pore radius of the skin. RESULTS AND DISCUSSION DOSE-DEPENDENT PENETRATION OF SDS INTO THE EPIDERMIS We first investigated whether increasing the total SDS concentration in the contacting solution above the CMC led to an increase in the concentration of SDS measured in the epidermis. An increase in the SDS concentration in the epidermis could explain much of the skin irritation data reported in the literature, since a larger "dose" of the SDS irritant in the skin would lead to greater skin irritation. Figure 1 shows that as the concentration of SDS in the contacting solution increased from 8.7 mM (the CMC of SDS) to 200 mM (about 20 times the CMC of SDS), the concentration of SDS measured in the epidermis increased in a linear manner, similar to the increased penetration reported by others (28,29). This finding clearly contradicts the monomer penetration model, according to which the amount of SDS penetrating into the epidermis should Table I Concentrations of SDS and PEO Used in the Dynamic Light-Scattering Experiments to Minimize the Concentrations of Free SDS Micelles and Free PEO Molecules in the Scattering Solution Concentration of SDS (mM) Concentration of PEO (wt%) 10 0.14 20 0.29 30 0.45 4O O.6O
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)