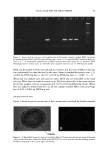
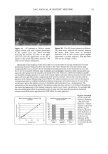
34 JOURNAL OF COSMETIC SCIENCE 12 :i• 6 o 4 o 2 CMC I 0 50 100 150 200 SDS Concentration in the Contacting Solution (mM) Figure 1. Effect of increasing the SDS concentration in the contacting solution on the amount of SDS that penetrates into the epidermis. The vertical dashed line indicates the CMC of SDS (8.7 mM), and the solid line through the data points is shown to guide the eye. The error bars reflect a 95% confidence interval based on six samples at each SDS concentration. remain constant as the SDS concentration increases beyond the CMC. Instead, Figure 1 vividly demonstrates that SDS present in miceliar form must be contributing to SDS penetration into the epidermis, since the SDS monomer concentration is constant over the experimental range examined, while the concentration of SDS micelies is increasing. The concentration of SDS in the epidermis measured here (2-9 wt%) compares well with the concentrations reported by others (4.3 wt%) (45). This large partitioning of SDS into the skin has been viewed as reflecting the binding of SDS molecules to the keratin protein in the corneocytes found in the SC (28,29,45). A mass balance of the amount of SDS found in the epidermis relative to the initial amount of SDS indicates that less than 1% of the SDS in the initial contacting solution penetrates into the skin for all the contacting solution used in Figure 1. Accordingly, one can assume that the contacting solution provides an infinite reservoir of SDS for penetration into the skin. Although the results in Figure 1 contradict the monomer penetration model, they do not contradict many observations reported in the literature where a surfactant dose- dependent skin irritation response was observed (1-3,7,10,13,15). Moreover, if the surfactant-induced damage to the skin is related to the actual amount of SDS in the skin, then the increased concentration of SDS in the epidermis shown in Figure 1 can be related to the increased skin irritation induced by SDS, observed by many researchers, as the SDS concentration in the contacting solution is increased (1-3,7,10,13,15). EFFECT OF PEO ON THE PENETRATION OF MICELLAR SDS INTO THE EPIDERMIS Having demonstrated that the amount of SDS penetrating into the epidermis above the
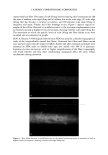
EPIDERMAL PENETRATION OF SDS MICELLES 35 CMC is related to the total SDS concentration (miceliar and monomeric) in the con- tacting solution, instead of only to the SDS monomer concentration, we examined the effect of adding PEO to the contacting solution. PEO is known to form micelie-like complexes with SDS, with the PEO forming a corona around the SDS micelies (35,38,46). The critical aggregation concentration (CAC) is the surfactant concentration at which polymer-bound micelies first form, in our case PEO-bound SDS micelies, and is lower than the CMC of the surfactant in the absence of PEO (39). When PEO is mixed with a solution of SDS, if there is an excess of PEO, the SDS monomer concentration is approximately equal to the CAC. Consequently, we anticipated that the contribution of the SDS monomers to skin penetration would be diminished in the SDS-PEO solutions because the SDS monomer concentration would be lowered. However, it was unclear how the SDS in the PEO-bound SDS micelies would behave when exposed to the skin, namely, whether this SDS could contribute to SDS penetration into the epidermis. Initially, we prepared solutions containing either 50 mM or 100 mM SDS as well as 2.0 wt% PEO. According to a molecular-thermodynamic theory developed recently (39), there is an excess of PEO at the two SDS concentrations examined. As a result, any micelies that form are bound to the PEO, and the SDS monomer concentration should be approximately equal to the CAC. Moreover, for PEO having a molecular weight of 8000 g/mol, we would expect, according to the theory (39), that there would be a maximum of 1 SDS micelie per PEO molecule. Figure 2 shows how the concentration of SDS measured in the epidermis was affected by the presence of PEO. Two aspects of Figure 2 should be noted: i. The addition of PEO at a given SDS concentration (50 mM or 100 mM) in the contacting solution leads to a significant reduction in the concentration of SDS measured in the epidermis. ii. The difference between the concentrations of SDS in the epidermis at 50 mM and • 6 •-•'4 0.•_ 0 3 I: 2 I: 1 0 0 ii I SDS SDS+PEO 50 mM 50 mM SDS , SDS+PEO 100 mM 100 mM Figure 2. Effect of adding 2 wt% PEO to a solution of SDS (50 mM and 100 mM) on the amount of SDS that penetrates into the epidermis. The empty bars indicate SDS penetration in the absence of PEO, and the solid bars indicate SDS penetration in the presence of 2 wt% PEO. The error bars reflect a 95% confidence interval based on six samples at each condition.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)