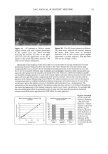
6 JOURNAL OF COSMETIC SCIENCE Table II Number of Surviving Microorganisms (CFU/g) in Periods of Time Specified by Official Methods for One Cosmetic Formulation Without Preservative and Eight Others With Different Preservative Systems Time Formula Organism 0 48 h 7 d 14 d 21 d 28 d S. putrefaciens B. cepacia E. coli S. aureus Bacillus sp. S, putrefaciens B. cepacza E. coli S, aureus Bacillus sp. S, putrefaciens B, cepacza E. coli S. aureu. g Bacillus sp. S. putrefaciens B, cepacza E. coli S. aureus Bacillus sp. S. putrefaciens B. cepacza E. coli S. aureus Bacillus sp. S. putrefaciens B. cepacza E. coli S. aureus Bacillus sp. S. putrefaciem B, cepacza E. coli S. aureus Bacillus sp. S. putrefaciens B. cepacza E. coli S. aureus Bacillus sp. S. putrefaciens B. cepacza E. coli S. aureu$ Bacillus sp. 4.2 x 10 6 2.0 x 10 6 3.2 x 10 6 4.50 x 105 2.20 x 10 4 1.70 x 10 2 4.0 x 106 7.4 x 106 1.77 x 106 2.20 x 105 5.7 x 104 2.2 x 10 2 3.4 x 106 5.5 x 106 2.32 x 106 1.00 X 10 4 2.50 X 103 3.00 X 102 1.31 X 107 9.6 X 106 1.00 X 107 5.4 X 105 4.0 X 103 10 1.08 X 106 6.2 X 103 3.5 X 103 4.2 X 103 5.0X 102 3.80X 102 3.5 x 10 6 10 10 10 10 10 4.7 x 106 5.6 x 103 10 10 10 10 6.0 x 10 6 10 10 10 10 10 1.17 x 10 7 1.02 x 103 30 10 10 10 1.9 x 10 6 4.7 x 10 3 3.4 x 103 3.8 x 10 3 2.50 x 10 2 2.80 x 10 2 2.93 x 106 5.10x 103 10 10 10 10 4.8 x 106 4.4 x 105 3.5 x 104 4.2 x 103 6.2 x 102 10 1.32 x 107 9.5 x 104 30 10 10 10 2.80 x 107 3.0 x 103 10 10 10 10 1.02 x 106 7.1 x 103 4.4 x 103 1.3 x 102 1.10 x 102 1.50 x 102 7.0 x 10 6 10 10 10 10 10 4.3 x 10 6 1.38 x 105 2.10 x 10 2 10 10 10 3.73 x 107 8.6 x 102 10 10 10 10 1.57 x 107 10 10 10 10 10 1.09x 106 4.7 x 103 4.9x 103 5.2 x 103 2.30x 102 2.10x 102 1.11 x 107 10 10 10 10 10 6.4 x 10 6 1.19 x 105 5.6 x 10 4 3.2 x 10 4 2.30 x 103 1.10 x 10 2 8.6 x 10 6 8.7 x 10 • 10 10 10 10 9.7 x 106 10 10 10 10 10 1.58 x 106 4.5 x 103 3.1 x 103 4.5 x 102 3.8 x 102 2.20 x 102 3.9x 106 10 10 10 10 10 7.8 x 106 9.8 x 103 6.4 x 103 3.4 x 102 10 10 1.24 x 107 10 10 10 10 10 7.8 x 106 10 10 10 10 10 3.0 x 106 4.2 x 103 6.5 x 103 1.90 x 103 5.4 x 102 3.9 x 102 2.32 x 106 10 10 10 10 10 3.4 x 106 4.3 x 105 10 10 10 10 9.6 x 106 10 10 10 10 10 2.93 x 107 40 10 10 10 10 2.30 x 105 4.1 x 103 4.3 x 103 4.5 x 10 2 3.4 x 10 2 2.50 x 10 2 3.0 x 106 10 10 10 10 10 3.0 x 106 10 10 10 10 10 5.3 x 10 6 10 10 10 10 10 1.02 x 106 10 10 10 10 10 2.90 x 105 1.1 x 103 5.2 x 103 6.2 x 103 4.6 x 102 2.10 x 10 2 6.6 x 105 10 10 10 10 10 3.8 x 10 6 10 10 10 10 10 9.8 x 10 6 10 10 10 10 10 9.9 x 106 10 10 10 10 10 1.12 x 105 8.4 x 103 6.1 x 103 6.5 x 103 4.3 x 102 3.3 x 10 2
PRESERVATIVE EFFICACY TESTING 7 self-sterilization of 10 6 CFU/ml is 24 hours. Considering the daily use of cosmetics, this specification may be appropriate. The self-sterilization periods obtained through D- values were confirmed by the experimental data, as they allow us to foresee if the preservative system is able to meet the requirements of the official methods by calcu- lating the rate of reduction after 7, 14, 21, and 28 days of the test. CONCLUSION In spite of the critics of the linear regression method, the method proved to be useful in the selection of an ideal preservative system for the evaluated product. However, before defining a system, one may want to consider confirming suitability using an official method. Thus, it is possible to conclude that among all the tested systems, the one used in formula 9 achieved the best results. REFERENCES (1) British Pharmacopoeia (Her Majesty's Stationary Office, London, 1998). (2) "Microbiological Tests, Antimicrobial Preservatives--Effectiveness," in United States Pharmacopeia XXIV (USP Convention, Rockford, MD, 2000). (3) "General Texts, Efficacy of Antimicrobial Preservation," in European Pharmacopeia, Third Edition (Coun- cil of Europe, Strasbourg, 1997). (4) "A Guideline for the Determination of Adequacy of Preservation of Cosmetics and Toiletry Formu- lations, in CTFA Technical Guidelines, C. N. McEwen and A. S. Curry, Eds. (Cosmetic, Toiletry and Fragrance Assn., Washington, D.C., 1993). (5) D. S. Orth, Linear regression method for rapid determination of cosmetic preservative efficacy, J. Soc. Cosmet. Chem., 30, 321-332 (1979). (6) D. S. Orth, Establishing cosmetic preservative efficacy by use of D-value, J. Soc. Cosmet. Chem., 31, 165-172 (1980). (7) D. S. Orth and L. R. Brueggen, Preservative efficacy testing of cosmetic products--Rechallenge test- ing and reliability of the linear regression method, Cosmet. Toiletr., 97, 61-65 (1982). (8) D.S. Orth, C.M. Lutes, S. R. Milsrein, and J.J. Allinger, Determination of shampoo preservative stability and apparent activation energies by the linear regression method of preservative efficacy testing,d. Soc. Cosmet. Chem., 38, 307-319 (1987). (9) D. S. Orth, Standardizing preservative efficacy test data, Cosmet. Toiletr., 106, 45-51 (1991). (10) D. S. Orth, R. F. Barlow, and L. A. Gregory, The required D-value--Evaluating product preservation in relation to packing and consumer use/abuse, Cosmet. Toiletr., 107, 39•43 (1992). (11) D. K. Brannan, Cosmetic preservation,J. Soc. Cosmet. Chem., 46, 199-220 (1995). (12) S. V. W. Sutton, R.J. Franco, M. F. Mowrey-McKee, S.C. Busschaert, J. Hamberger, and D. W. Proud, The D-value determinations are an inappropriate measure of disinfecting activity of common contact lens disinfecting solutions, Appl. Environ. Microbial., 57, 2021-2026 (1991). (13) G. E. Borovian, Pseudomonas cepacia: Growth in and adaptability to increased preservative concentra- tions, J. Soc. Cosmet. Chem., 34, 197-203 (1983). (14) J. Close and P. A Nielsen, Resistance of a strain of Pseudomonas cepacia to esters of p-hydroxy-benzoic acid, Appl. Environ. Microbiol., 31,718-722 (1976).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)