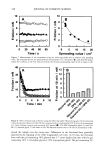
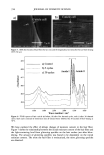
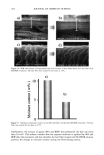
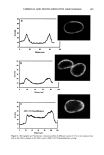
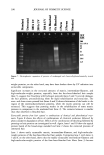
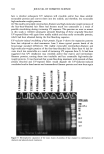
j. Cosmet. sci., 54, 379-394 (July/August 2003) Chemical and photo-oxidative hair damage studied by dye diffusion and electrophoresis S. B. RUETSCH, B. YANG, and Y. K. KAMATH, TRI/Princeton, 60I Prospect Ave., Princeton, NJ 08542. Accepted for publication September 6, 2002. Synopsis Microspectrophotometric and electrophoretic methods were used to characterize and quantify the effects of primary damage to hair from chemical and photochemical oxidative processes. The diffusion of molecules proceeding from the fiber surface to the center of untreated and modified (by chemical and photochemical oxidative processes) hair fibers was mapped by fluorescence microscopy and quantified by calculating diffusion coefficients of a fluorescent molecule. In addition, an electrophoretic separation technique, namely, SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis), was used not only to substantiate the results obtained in the microfluorometric study, but also to show how the main classes of proteins of unaltered hair are modified by cosmetic chemical treatments, light exposure, and combinations of these two processes. UV microspectrophotometry is an alternate analytical method to evaluate photo-oxidative damage in hair, and supports the results obtained by microfluorometry. INTRODUCTION Natural weathering and grooming practices to improve appearance inflict irreversible damage to human hair. Grooming practices such as combing, blow drying, and brushing lead to mechanical damage, mainly to the surface of the hair fiber. On the other hand, chemical methods such as bleaching, perming, and photochemical oxidation result in chemical damage to the cuticle and cortex. The nature of this damage is in the form of cleaved chemical bonds, which are further oxidized to hydrophilic (acidic) functional- ities. This alters the properties of the material, such as the extent of swelling and receptivity to other molecules that interact strongly with the acidic functionalities. In undamaged hair, diffusion of dyes with a molecular weight of-300-400 is relatively difficult and requires more than one hour to penetrate through the cuticular layers to reach the cortex. The molecular architecture within the cell structure restricts access to foreign molecules such as dye molecules. In chemically damaged fibers, on the other hand, cleavage of the disulfide bonds and further oxidation decrease the disulfide crosslink density in the matrix, and result in the formation of hydrophilic sulfonic acid groups. In some cases, damage can also result in cleavage of the peptide bonds and formation of carboxyl and amine groups, both of which are hydrophilic. The overall effect of such damage is an increase in swelling of the hair fiber (in the wet condition), 379
380 JOURNAL OF COSMETIC SCIENCE which in turn, results in improved access to dye molecules, and can be characterized by the diffusion coefficient. Therefore, the diffusion coefficient of a selected dye molecule (under specific dyeing conditions) can be used as a quantitative measure of the damage inflicted upon the fiber. Such methods are used in characterizing changes in the structure of synthetic fibers subjected to different processing methods (1). This study investigates the effects of chemical and photochemical oxidative processes on (a) the microstructure of human hair and (b) the proteins from different histological components of hair. Using microfluorometry, oxidative damage is characterized and quan- tified by studying the changes in diffusion kinetics of the fluorochrome uranine into the hair shaft. The results of the UV radiation-induced photo-oxidative damage to the keratin structure are compared with those obtained in our earlier studies (2) with chemically bleached hair. Increased dye diffusion rates are indicative of changes in fiber morphology. An electrophoretic separation technique was used to show how the main classes of proteins of unaltered hair are modified by cosmetic chemical treatments, light exposure, and com- binations of these processes. The molecular weights of the extractable main classes of proteins of unaltered as well as chemically and photochemically altered hair were es- tablished. Decreases or increases in the amounts of extractable proteins relative to untreated hair suggest which proteins were modified by these chemical/photochemical treatments. Occurrence of new protein bands not observed in untreated hair is indicative of the treatment-induced breakdown of proteins, which were originally not extractable. On the other hand, the absence of protein bands in treated hair, which were observed in untreated hair, suggests further crosslinking of the protein network, which makes them less soluble and therefore less extractable. EXPERIMENTAL HAIR SAMPLES Dark brown hair from DeMeo Brothers, New York, was used. CHEMICAL OXIDATION Bleaching was carried out by two different methods. In one method, hair was bleached for one and four hours with 6% alkaline hydrogen peroxide at room temperature (the pH was adjusted to 10.2 with ammonium hydroxide). The bleaching solution was freshly replaced every 30 minutes. In the other method, hair fibers were treated for 30 minutes with a bleach cream containing hydrogen peroxide and ammonium persulfate (pH 10.2). All samples were thoroughly rinsed, air-dried, and then placed in a desiccator for 24 hours. PHOTOCHEMICAL OXIDATION--EXPOSURE TO SOLAR-SIMULATED UV RADIATION Individual hair fibers were mounted in parallel on templates and exposed for a total of 100,200, 300, 500, and 600 hours to alternating three-hour cycles of UV radiation and humidification as in a QUV accelerated weathering tester. This unit simulates the sunlight in the range of 290-400 nm, with an irradiance maximum at 340 nm. The
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)