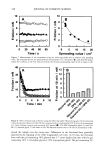
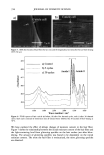
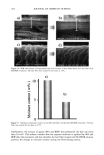
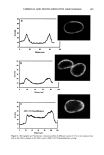
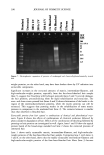
j. Cosmet. Sci., 54, 395-409 (July/August 2003) New insights into the physicochemical effects of ammonia/peroxide bleaching of hair and Sepia nlelanins PADMAJA PREM, KATHERINE J. DUBE, STEPHEN A. MADISON, and JOHN BARTOLONE, Hair Colorants Division, Unilever Research and Development, 45 River Road, Edgewater, NJ 07020. Accepted for publication September 27, 2002. Synopsis Chemically unaltered melanosomes from black hair were isolated using a mild enzymatic procedure reported by Novellino et al. (1) involving sequential treatment of a homogenized hair sample with different protease enzymes. Time-dependent fluorescence studies show, under identical conditions, that the rate of bleaching upon NH3/H202 treatment of hair melanosomes is twice that of Sq ia melanosomes. The structure and morphology of hair melanosomes are compared to Sepia eumelanin using ESEM and TEM imaging studies. Black hair melanosomes are aggregates of rice-shaped ellipsoidal particles (0.8-1.0 pm in length and 0.2-0.6 pm in width) surrounded by an amorphous material suspected to be made of non-proteinacious materials. Sepia eumelanin aggregates are larger (2-5 pm) particles with a "doughnut" shape comprised of 100-150-nm spherical particles. Time-dependent TEM imaging studies of ammonia-treated (pH 10) hair melanosomes showed an initial breakdown of melanosomal aggregates followed by rupture of the melano- somal membrane, releasing melanin nanoparticles and leaving a ghost membrane behind. After prolonged treatment with aqueous NH3, a total loss of characteristic melanosome morphology was observed leading to an amorphous material. By contrast, Sepia melanosomes under identical conditions of ammonia treatment did not show such changes, probably due to different surface properties and aggregation behavior. Sodium hydroxide or sodium carbonate at identical pH did not show similar changes to ammonia, suggesting that the changes are not merely due to alkaline pH, but, rather, are specific to ammonia. Co-treatment with ammonia and peroxide induced a faster disintegration of the melanosomes, resulting in a complete disso- lution and discoloration of melanin in 30 minutes. The data suggest that ammonia helps to release melanin nanoparticles out of melanosomes, making them more susceptible to oxidative attack by 8202. INTRODUCTION Melanins are a class of highly heterogeneous biological pigments found in animals, plants, and humans, and are responsible for the color of skin, eyes, and hair. The color of hair and wool in mammals and feathers in birds is mostly determined by the quantity and quality of melanin that is synthesized in follicular melanocytes and transferred to keratinocytes. It is generally known that hair pigmentation is due to two types of Address all correspondence to Padmaja Prem. 395
396 JOURNAL OF COSMETIC SCIENCE melanin: black-to-brown eumelanin and yellow-to-red pheomelanin (2). Eumelanin is believed to be a polymer derived from oxidative copolymerization of 5,6-dihydroxyin- dole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA) (3,4). Pheomelanin is composed of tyrosine and cysteine-derived units constructed into benzothiazine mono- mers that make the polymer (5). Extensive research work has been conducted by various groups to elucidate the structure of melanin and the melanogenesis pathway. Despite the vast literature on natural and synthetic melanins, the molecular structure of this class of pigments remains unknown. This is due to the lack of adequate methods to isolate melanin from biological sources, their insolubility at neutral pH, and the heterogeneity in their structural features. Melanins are typically isolated under harsh hydrolytic con- ditions such as prolonged treatment with concentrated hydrochloric acid or sodium hydroxide. Recent studies demonstrate that melanin, when exposed to boiling mineral acids, suffers profound structural alterations, particularly extensive decarboxylation (3,6). The isolation of melanin from hair or fur is more complex because of the com- pactness of the keratin in which the pigment is encapsulated. As a result, most of the literature work used Sepia melanin or synthetic eumelanin as a model for black hair melanin. Regardless of the source and type of melanin, various physical and chemical methods have been established in the last fifty years towards characterizing the structure of eumelanin and pheomelanin at the microscopic and macroscopic levels. Both X-ray and mass spectroscopic studies gained insight into the structural units of eumelanin and suggest that synthetic eumelanins and natural eumelanin from Sepia have similar build- ing blocks (7-9). Based on wide-angle X-ray diffraction measurements of eumelanin, a fundamental particle consisting of a graphite-layered structure with four to eight con- nected monomers per layer has been proposed. Both MALDI-mass spectroscopy and X-ray diffraction studies identified oligomeric units of masses in the range of-500- 1000 amu for Sepia eumelanin and synthetic eumelanin (8-10). Scanning electron mi- croscopy studies of size- selected Sepia melanin samples suggest that the pigment of eumelanin is an aggregated structure with subunits of-150 nm, and that smaller -15-nm-size particles adhere to these larger subunits (11). Recent AFM studies on Sepia melanin also confirmed that the 100-200-nm-size spherical particles are not a funda- mental structural unit of eumelanin, but are composed of smaller 5-15-nm-size particles (12). A review by Prota (13) claims the color of black hair is due to intact or poorly degraded eumelanin, whereas brown hair contains a degraded variant of eumelanin. Prota also claims that only red hair types contain pheomelanins. The study by Borgers et al. (14) uses sensitive HPLC methods for the identification and quantification of eumelanin and pheomelanin from human hair by measuring product yields produced by alkaline hy- drogen peroxide degradation of melanin. This study suggests that color determination for black, brown, or blonde hair depends more on melanin quantity than on eu-/ pheomelanin composition, while red hair may arise through production of pheomelanin. Wolfram and Albrecht (15) studied the oxidation of human hair with and without melanin pigment and the oxidation of melanin granules isolated from human hair. They found that hair with pigment degrades hydrogen peroxide at a much faster rate than hair without the pigment. Thus, they conclude that peroxide reacts with melanin much faster than with proteins. In another study, the same authors compared the chemical and photobleaching of brown and red hair and concluded that pheomelanin is more resistant
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)