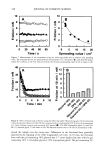
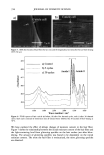
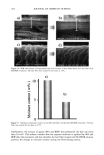
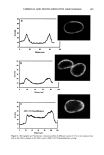
CHEMICAL AND PHOTO-OXIDATIVE HAIR DAMAGE 381 irradiance intensity factor was chosen to be 1.35, compared to 1.0 for regular sunlight. The energy density at the 340-nm wavelength was kept constant at 0.96 W/m 2. The total energy density in the wavelength range of 300-400 nm is 5.06 W/m 2. MICROFLUOROMETRIC STUDY Dyeing with uranine. The photo-oxidized hair fibers as well as the untreated controls were dyed in a 0.1% aqueous uranine solution (pH 7) at 50øC for 5.5 hours. The dyed fibers were rinsed thoroughly for several minutes in warm, distilled water, air-dried, and placed in a desiccator over P205 for 24 hours. Sample preparation for microfluorometry. The dried, dyed fibers were embedded in Spurr's low viscosity resin, cured for 24 hours at 70øC, microtomed at 10-1•m thickness, and viewed in a Leitz MPV 1. ! microspectrophotometer with the Ploem vertical illuminator in the narrow-band blue excitation beam. Spectral scans were obtained on longitudinally viewed fibers, and the wavelength of maximum fluorescence (k,•) was established at 540 nm. Spatial scans were made across the 10-pm-thick fiber cross sections at the established k,, (540 nm). Instrumental settings for spectral and cross-sectional scans ß Blue excitation: 450•490 nm KP: 510 nm LP: 515 nm ß Objective: 25x for spectral scans 40x for cross-sectional scans ß Wavelength, k,,: 540 nm for cross-sectional scans ß Measuring sensor: 20 x 30 pm 2 for spectral scans 3.1 x 25.0 pm 2 for cross-sectional scans ß Accelerating voltage: 1.6 kv ß Scanning speed: 7.2 pm/s Micrographs of the cross-sectional views were made with Kodak slide film at 160 ASA at 60-seconds exposure time, using a 25x objective, 1.6x collar, and a 10x ocular). ELECTROPHORETIC STUDY Hair samples/treatments. Hair samples with treatment sequences were: ß Untreated ß Untreated 100 h/P00 h/300 h UV ß Bleached 1 h/4 h with alkaline 6% H202 ß Bleached 1 h/4 h as above 100 h/300 h UV ß Bleached 1 h/4 h H202/300 h UV/bleached 1 h H202 ß Permed lx/3x ß Permed 1 x/3x as above 100 h/300 h UV ß Bleached/permed ß Bleached/permed/300 h UV Extraction of hair proteins. From each of the above listed categories, 5 to 10 mg of 5-mm-long hair segments were immersed in an extraction buffer containing 0.05 M dithiothreitol (DTT) as reductant, 8 M urea as denaturing agent, and 0.05 M Tris (hydroxymethyl) amino-methane. The ratio of hair to extraction buffer was 1:100. The samples were extracted for 24 hours at ambient temperature, and finally sonificated for 30 minutes. The following reaction with DTT helps to open the keratin matrix to
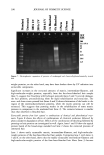
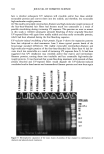
382 JOURNAL OF COSMETIC SCIENCE facilitate the diffusion of solubilized protein out of the hair fiber. The reduction reaction is given as: K-S-S-K + HS-(CH2)4-SH •=• 2 K-SH + (CH2)4-S-S-(CH2) 4 where K = keratin. Derivatization of thiols. Derivatization was done according to the equation given below with 20% iodoacetamide for 30 to 60 minutes while sonicating at -40øC. The ratio of extraction buffer/derivatization solution was 10:1. The samples were then centrifuged, and the supernatant liquid (containing the extracted/derivatized proteins) was taken and placed into new vials for either freezing or instant use. K-SH + I-CH2-CONH 2 • K-S-CH2-CONH 2 + HI where K -- keratin. SDS electrophoretic separation technique. SDS (sodium dodecyl sulfate)-PAGE (polyacryl- amide gel electrophoresis) separates proteins exclusively according to their molecular weight. The proteins are loaded with the anionic detergent SDS, about 1.4 g SDS per 1 g protein, and therefore, build SDS-protein micelle complexes with approximately a constant net charge per mass unit. Therefore, all SDS-protein micelie complexes are highly negatively charged and rapidly migrate towards the anode, which leads to rapid separation. Therefore, it is important to note that this separation is based primarily on molecular weight, because SDS protein micelle complexes have a similar characteristic net charge per mass unit. The extracted, denatured, and derivatized proteins, now unfolded into polypeptide chains, are entered into the wells of the gel alongside the protein standard of known molecular weight (up to -200 kD). When the smallest protein has traveled nearly to the bottom of the gel, the electrophoretic run is stopped. The gel is removed from the chamber and then stained and destained, causing the stained gel to become lighter, but leaving a series of bands indicating the presence of separated proteins. The gel is then scanned into the computer for recording and enhancement of the digital image. The relative intensity of the bands in various lanes of the gel indicates the relative amount of each extracted protein. The molecular weights of the unknowns were established by comparing their relative electrophoretic mobility with those of the protein standard. The relative distance the protein travels down the gel is directly related to the log of its molecular weight. The greater the molecular weight, the smaller the relative electro- phoretic mobility of the protein and the shorter the relative distance traveled down the gel, and vice versa. Microcrofluorometric scans along the bands in the various lanes of the gel can be made to quantify the relative amount of each of the extracted proteins. Quantitative compari- sons can be made. RESULTS AND DISCUSSION MICROFLUOROMETRY Background. Dye diffusion rates in keratin are strongly affected by changes in fiber morphology (3). Therefore, dye diffusion rates are used to quantify damage to the hair fiber by oxidative processes. Oxidative processes are known to decrease the disulfide
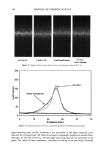
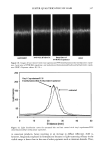
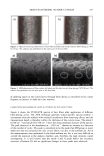
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)