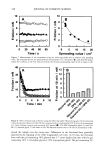
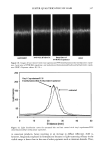
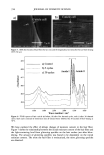
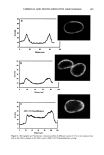
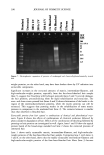
CHEMICAL AND PHOTO-OXIDATIVE HAIR DAMAGE 389 Absorbance 0,64 O. 4B 0.32 24.0 48.0 72.0 g6.0 t20.0 Distance ( gm ) Absorbance O.EiO 0.64 O. 48 0.32 0.t6 -b .0 , I , I I I I I .0 24.0 48.0 72.0 96.0 I t20 Distance ( gm ) Figure 6. Typical cross-sectional scans across stabilizer-free hair fibers (a) before and (b) after UV exposure. (Scans were carried our ar Xo, = 340 nm, the absorbance maximum of photodegradation products in hair.) inhibited after long-term UV radiation (lane 5 = 300 h). However, a faint protein band develops after 300-h UV exposure in the region of low electrophoretic mobility, which indicates extraction of very small amounts of a high-molecalar-weight protein. The mo- lecular weight of this protein is more than 100 kDa. These features suggest that UV radiation may crosslink the matrix and ir•termediate-filament proteins, turn them into insoluble high-molecular-weight proteins, and inhibit their extraction. High-molecular-
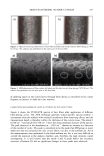
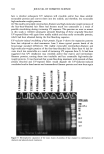
390 JOURNAL OF COSMETIC SCIENCE I 2 3 4 5 6 7 8 9 I0 MW 200 116 / 97.4 45 31 21.$ 14.4 6.$ Figure 7. Electrophoretic separation of proteins of undamaged and chemically/photochemically treated hair. weight proteins, on the other hand, may have been broken down by UV radiation into extractable components. Significant increases in the extracted amounts of matrix, intermediate-filament, and high-molecular-weight proteins, especially from the four-hour-bleached hair sample (lane 7), suggest that bleaching with hydrogen peroxide (lanes 6 and 7) severely damages the hair proteins, preconditioning them for rapid solubilization and extraction. The once- and three-times-permed hair (lanes 8 and 9) shows elimination of the bands in the region of the intermediate-filament proteins, while the matrix proteins can still be extracted. This suggests that perming results in the solubilization of some matrix proteins in comparison to the unaltered hair (lane 2). The extracted amount is smaller than that observed in bleached hair. Extractable proteins from hair exposed to combinations of chemical and photochemical treat- ments. Figure 8 shows the effects of combinations of chemical oxidation followed by photo-oxidative degradation of hair. Effects of the combination of bleaching followed by perming on hair proteins are investigated as well. Again, lanes 1 and 10 show the typical protein bands of the broad-range standard and lane 2 shows the extracted proteins of untreated hair. Lane 3 shows easily extractable matrix, intermediate-filament, and high-molecular- weight proteins of the four-hour-bleached hair sample. Comparing lane 3 with lanes 4, 5 and 6, on the other hand, shows that the readily extractable intermediate-filament and high-molecular-weight proteins of the four-hour-bleached hair fibers (lane 3) become
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)