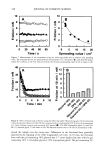
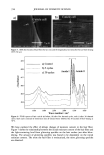
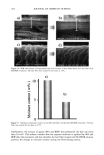
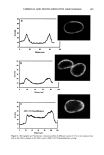
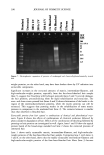
AMMONIA/PEROXIDE BLEACHING OF HAIR 409 (11) J. B. Nofsinger, S. E. Forest, K. A. Gold, and J. D. Simon, Pig. Cell. Res., 13, 179-184 (2000). (12) C. M. R. Clancy, J. B. Nofsinger, and J. D. Simon, J. Phys. Chem. B., 104, 7871-7873 (2000). (13) G. Prota, Pig. Cell. Res., 13, 283-293 (2000). (14) C. R. Borgers, C. J. Roberts, D. G. Wilkins, and D. E. Rollins, Analyt. Biochem., 290, 116-125 (2001). (15) L.J. Wolfram and L. Albrecht, J. Soc. Cosmet. Chem., 37, 294 (1986). (16) L.J. Wolfram and L. Albrecht, J. Soc. Cosmet. Chem., 38, 179-191 (1987). (17) J. M. Gallas, G. W. Zajac, T. Sarna, and P. L. Stotter, Pig. Cell. Res. 13, 99-108 (2000). (18) P. Kayatz, G. Thumann, T. T. Luther, and J. F. Jordan, Invest. Ophthalmol. Vis. Sci., 42, 241-246 (2001). (19) J. A. Swift, Nature, 203, 976-977 (1964).
j. Cosmet. sci., 54, 411-420 (July/August 2003) The preservative efficacy testing method for powdered eye shadows M. R. S. E. L. SOUZA and M. T. OHARA, Rua da Consola•'•o 3064, Apto. 62A, 01416-000 S•7o Paulo, Brazil. Accepted for publication January 13, 2003. Synopsis Preservative efficacy testing is based on a sample inoculation using a microbial suspension with a determined amount of colony-forming units (CFU). After that, the number of survivors is investigated by periodic evaluations, and the results are compared with specifications. When liquid cosmetics are evaluated, it is easy to obtain homogeneity between the inoculum and the sample, but for a powder sample it cannot be guaranteed. In this context, freeze-dried microorganisms could be used to help the homogenization. In this research, the preservative efficacy is evaluated using a powdered eye shadow. The microorganisms used were Staphylococc•s aureus, Pseudomonas aowginosa, Candida albicans, and Aspergillus niger. The challenge tests were performed in samples with (P) or without (NP) added preservative. The methods used to evaluate the results were the ones described in the official compendia and in the cosmetics guides of international associations, also using linear regression in calculating the D-value. The results showed that it is possible to use freeze-dried microorganisms instead of suspension to evaluate the preservative efficacy of cosmetic solids. The microorganism stability was verified by the determination of the microbial load and the minimum inhibitory concentration after freeze-drying and during the following six months. INTRODUCTION Preservative efficacy testing is an essential part of substantiating the safety of cosmetic products. The correct use of preservatives protects the product against contamination while it is in trade channels and in the hands of the consumer (1-4). When consumers use cosmetic products, they repeatedly challenge the cosmetic with microorganisms in saliva, on dirty hands, and in tap water (5). In this context, preservative efficacy must be evaluated to assure the product's safety. The methodology for the evaluation of preservative adequacy is described in the official compendia for pharmaceuticals (6-9). It is also given in cosmetic guides such as those of the Cosmetic, Toiletry and Fragrance Association (CTFA) (10) and the American Society for Testing and Material (ASTM) (5). All methods are applied to liquid and semi-solid products, and they are based on the challenge test, which consists in the contamination of the product by fresh pure cultures of microorganisms, suspended in saline, followed by periodic evaluations. The CTFA guidelines (10) describe methods for testing eye area cosmetics. The inocu- 411
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)