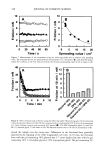
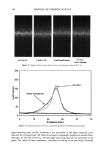
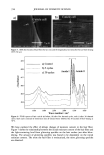
AMMONIA/PEROXIDE BLEACHING OF HAIR 399 D.?• - Figure l. Absorption spectra of aqueous suspensions of hair and Sepia melanin at pH 7.0. Sonicated suspensions of 0.1 mg/ml of melanosomes in water were used for the absorption spectral measurements. FLUORESCENCE PROPERTIES OF HAIR AND SEPIA MELANIN Both isolated hair melanosomes and Sepia melanin at pH 10.0 did not show any fluo- rescence before bleaching. However, after bleaching with NH 3 and H202 at pH 10, both melanins exhibited fluorescence properties. Figure 2 shows the fluorescence emission (}tex = 350 rim) and excitation spectra (}ter n = 450 rim) of hair melanin (0.5 mg/ml) after 45 min of treatment with 2% H202 and 0.75% NH 3 at pH 10.0. The emission maximum is at 468 nm for any excitation wavelength from 300-350 nm. No other emission band is observed for hair melanin. These results agree with the finding by Kayatz et aL (18) that both synthetic melanin and isolated bovine melanosomes fluoresce only after oxidation. However, the fluorescence emission band is reported to be at 548 nm for bovine melanosomes, while hair melanosomes did not show any fluorescence emission in this region. Figure 3 shows the emission and excitation spectra of Sepia melanin under identical conditions of treatment with ammonia and peroxide. The fluorescence emission spec- trum of Sepia melanin showed a maximum at 460 nm (}tex = 350 rim) and an excitation maximum at 349 nm (}tern = 450 rim). The excitation spectra of both Sepia and hair melanin exhibited maxima around the 350 nm region, suggesting that a yellow chro- mophore is responsible for the fluorescence emission at 460 nm. The rates of ammonia/peroxide bleaching of both Sepia and hair melanins were studied by time-dependent fluorescence measurements under identical conditions. Suspensions of 0.2 mg/ml of melanin samples were treated with 1% NH 3 and 2% H202 at pH 10.0 and 25øC. Oxidation of melanins results in fluorescence emission at 450 nm, as shown above. The intensity of fluorescence emission increases with increase in time. Figure 4
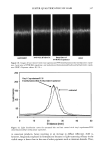
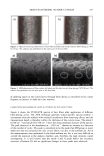
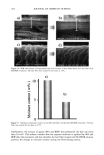
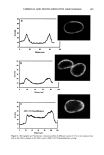
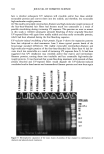
400 JOURNAL OF COSMETIC SCIENCE 3O 20 10 ø / 200 300 excitation ..-' \ ! \ i Wavelenõth {rim} Figure 2. Fluorescence emission and excitation spectra of isolated hair melanosomes (0.5 mg/ml) after 45 min of oxidation with 2% H202 and 0.75% NH3 at pH 10.0. Fluorescence intensity is shown in arbitrary units. shows the plot of fluorescence emission intensity at 450 nm as a function of time for both hair and Sepia melanosomes at pH 10.0. The rate of bleaching of hair melanin at pH 10 is 2.1 x 10 -4 s -1, nearly twice as fast as that of Sepia melanin, 1.2 x 10 -4 s -1, under identical conditions. The fluorescence emission intensity reached a saturation value after complete oxidation, resulting in a clear solution for hair melanin and a yellow solution for Sepia melanin. The rates of bleaching of Sepia and hair melanin are different, possibly due to differences in mor- phological and aggregation behavior of the melanosomes. These aspects were verified using ESEM and TEM imaging studies. ESEM AND TEM STUI)IES: COMPARISON OF SEPIA AND HAIR MELANOSOMES ESEM and TEM imaging studies were performed to understand the structural and morphological properties of isolated hair melanosomes. For comparison, Sepia melanin was also studied, since it is widely used as a model for black eumelanin. Figure 5 shows the ESEM image of a suspension of Sepia melanin in water. Sepia melanosomes are micron-sized particles with a characteristic "doughnut" shape. Magnification of the micron-size particles revealed that they are comprised of aggregates of nanometer-size particles (100-150 nm). It is also noted that the micron-size particles of Sepia melanin could be easily broken down to nanoscopic particles upon sonication. These results are consistent with the previous work by Nofsinger eta/. (11) on SEM imaging studies of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)