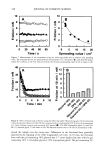
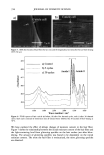
AMMONIA/PEROXIDE BLEACHING OF HAIR 405 Figure 7. TEM image of hair melanosomes after 30 rain of ammonia treatment at pH 10.0 (x75,000). A suspension of 0.05 mg/ml of hair melanosomes was treated with 1.0% ammonia at pH 10.0. and hair melanosomes did not show fluorescence before bleaching, while after oxidation with NH 3 and H202, the fluorescence properties of both melanins exhibited identical emission and excitation spectra. From the excitation spectra, it is clear that a yellow chromophore is responsible for the fluorescence emission properties of melanin after oxidation. This yellow choromophore could be indoquinone or similar quinoid struc- tures resulting from the breakdown and degradation of polymeric melanin as a result of oxidation. The fluorescence data also suggest that Sepia and human hair melanosomes have the same molecular constituents. Time-dependent fluorescence studies show that, under identical conditions, the rate of bleaching upon NH3/H20 2 treatment of hair melanosomes is twice that of Sepia melanosomes. The rates of bleaching of Sepia and hair melanin are different, possibly because of differences in morphological and aggregation behavior of the melanosomes. The morphological behavior of isolated hair melanosomes was compared to that of Sepia melanosomes using ESEM and TEM imaging studies. Black hair melanosomes are aggregates of rice-shaped ellipsoidal particles (0.8-1.0 torn in length and 0.2-0.6 tom in width) surrounded by an amorphous material suspected to be made of non-proteinacious
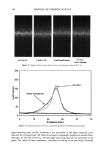
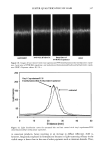
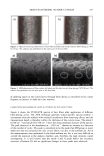
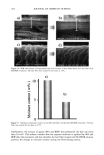
406 JOURNAL OF COSMETIC SCIENCE . : •... /.- . . ,• -.: ß .,,,.. ,.. ß -• ........ jl "•' :" ' 'i: • . . :j •... :.. x . '- .• .-• . . . ,. ,.f . .:.. ...z - ß ':' *'? .•. 't :,- ..•. .. . . . . .. .':, 'r.' ...---- • .. ! ..,,L',' '- • "• . .., :.. . Figure 8. TEM image of isolated hair melanosomes after 120 min of ammonia treatment at pH 10 (x75,000). A suspension of 0.05 mg/ml of hair melanosomes was treated with 1.0% ammonia at pH 10.0. materials. Since the isolation of melanosomes involved sequential treatments with dif- ferent protease enzymes, this amorphous material could not be ordinary proteins, but, rather, highly resistant glycolipids or glycoproteins. A report by Swift (19) indicates a closer association between melanin granules and the cell membrane complex in matured hair samples. Thus, the amorphous material seen in isolated melanosomes could possibly be the cell membrane complex. More work needs to be done to identify the nature of the waxy material surrounding the melanosomes. Sepia eumelanin aggregates are larger (2-5 pm) particles with a "doughnut" shape comprised of 100-150-nm spherical particles, and do not show the waxy material. The ESEM data on Sepia melanin correlates very well to the published SEM data by Nofsinger et al. (11). Magnification of the micron-size particles of Sepia melanosomes showed smaller particles of 100-150 nm in size, indicating that the bigger spherical particles are aggregates of smaller nanoparticles. The reported literature also confirms that the self- assembly of Sepia melanin is a hierarchical process (12). Similar subunits were not seen in isolated hair melanosomes under identical magnification, probably due to the fact that hair melanin nanoparticles are tightly encapsulated in a membrane sac that acts as a protective barrier. Time-dependent TEM imaging studies of ammonia-treated hair melanosomes help us to understand the early events occurring in the bleaching process of isolated hair melano-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)