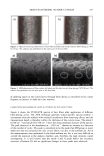
AMMONIA/PEROXIDE BLEACHING OF HAIR 397 than eumelanin to chemical or photodegradation (16). It is also known from the litera- ture that the ammonia/hydrogen peroxide combination is an effective melanin-bleaching agent, whereas hydrogen peroxide at neutral conditions bleaches melanin very slowly. Studies by Gallas et al. (17), using scanning tunneling microscopy (STM), showed a dramatic decrease in the STM-measured apparent heights for mildly bleached melanin compared to untreated melanin. These authors interpret the observed decrease as a result of oxidative conversion of the multilayered stacked sheets of melanin to largely destacked melanin sheets. There are no detailed studies in the literature on the ultrastructural features of hair meianosomes or on the mechanism of hair melanin bleaching induced by ammonia and peroxide. In order to study the mechanism of human hair melanin bleaching, intact hair melanosomes were isolated using an enzymatic procedure (1) to digest hair keratin under neutral conditions. The present study, aimed at understanding the mechanism of hair melanin bleaching, presents the first report on the direct demonstration by TEM of morphological and structural changes undergone by isolated hair melanosomes as a result of ammonia and ammonia/peroxide treatments. The study reveals that ammonia has a specific role in the bleaching process by breaking the melanosomal membrane, unlike other alkalizing agents such as sodium hydroxide and sodium carbonate at identical pH conditions. In addition, we also show by TEM that the micron-sized hair melanosomes are composed of smaller constituents of melanin (30-60 nm) encapsulated in a membrane sac. EXPERIMENTAL MATERIALS Untreated Asian black hair was purchased from International Hair Importers and Prod- ucts Inc., Bellerose, NY. Sepia melanin and all protease enzymes were purchased from Sigma Biochemicals (St. Louis, MO). All other chemicals and reagents used in the study were analytical grade. All solutions were prepared using milli-Q-water, and melanin suspensions were prepared freshly by sonication prior to the experiments. METHODS Isolation of hair eume/anin from Asian Mack hair. The isolation of black hair melanin was achieved by the procedure reported by Novellino et aL (1), using the following enzymatic treatments: (1) An Asian black hair sample, after removal of sebum and surface lipids, was treated with DTT (dithiothreitol) for 18 hr at pH 7.4 and 37øC. After centrifuga- tion, the pellet was treated with Proteinase K and DTT and stirred under argon at 37øC for another 18 hr. The mixture was centrifuged for 20 min (3500 x g, 4øC) in an Eppendorf centrifuge. (2) The pellet was extensively rinsed with water, suspended in 30 ml of 0.1 M phosphate buffer, and treated with papain and DTT at 37øC under argon for 18 hr. The mixture was centrifuged as above. (3) The black pellet collected after sixfold washing with water was suspended in 30 ml of buffer and treated with protease and DTT at 37øC under argon for 18 h. (4) The dark residue obtained after centrifu- gation was suspended in 40 ml of phosphate buffer deaerated with argon for 30 min. An oxygen-free solution of 2% Triton X-I00 was added and stirred at room temperature for 4 hr under argon. The mixture was centrifuged for 20 min (106,000 x g, 4øC). After
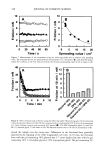
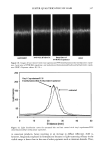
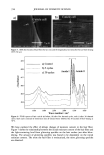
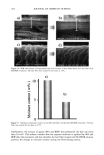
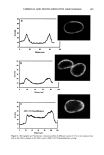
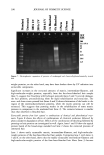
398 JOURNAL OF COSMETIC SCIENCE washing with water:methanol (1:1) and four times with water, the black pellet was again treated with protease and DTT as described in step 3. The final pigment collected by centrifugation was dried over NaOH overnight and equilibrated with saturated aqueous CaC12 for 24 hr. The final yield of melanin was 600 mg from 15 g of Asian black hair (4%). Environmental scanning electron microscopy stz/dies (ESEM). ESEM studies were conducted using the FEI ESEM (model E-3) equipped with a lanthanum hexaboride (LAB6) fila- ment. The microscope was equipped with a Peltier cooling stage (used to maintain the moist environment), which was operated at 2.0ø-3.0øC while the chamber pressure was maintained between 4 and 5 Torr. The sample chamber was pumped down to 5 Torr and then increased to 9 Torr. This was repeated eight times while maintaining a Peltier temperature of 2ø-3øC. Samples were then viewed at 10-24 kV while maintaining a chamber pressure of 4-5 Tort. Occasionally when the liquid precluded the viewing of melanin, the pressure was decreased to remove surface liquid and reveal the melanin. The ESEM was also equipped with the Image Acquisition and Archive System (IAAS), which allowed the results to be recorded and stored digitally as TIF files. Transmission electron microscopy studies (TEM). A Jeol 1200-EX transmission electron mi- croscope equipped with a tungsten filament was used for all imaging studies. The vacuum in the chamber was maintained at 10 3 Pa. Samples were viewed at 100 keV with a low beam current of 63-65 uA. An AMT digital camera system was attached to the port of the TEM. The images were captured, and the signal was sent to a PC for image storage and analysis. The specimen support grids used in the experiments were 200-mesh gold finder grids. Finder grids were used to provide an ease of relocation in interesting areas of the grid. The gold grids had a formvar support film and a carbon coat. The specimens were observed under the following sample preparation: A 5-1nl drop of melanosome suspension was placed on a formvar/carbon-coated gold grid. The sample was allowed to settle for one minute, excess liquid was gently drained off with filter paper, and the sample was allowed to dry. UV-Vis and fluorescence spectroscopic studies. UV-Vis spectral studies were performed using a Shimadzu Multispec-1501 UV-Vis spectrophotometer with diode array detection. Time-dependent spectral measurements were recorded in the scan mode. The fluores- cence studies were performed using a Cary Eclipse fluorescence spectrophotometer. RESULTS UV-VIS ABSORPTION PROPERTIES OF HAIR AND SEPIA MELANIN The absorption spectra of aqueous suspensions (0.1 mg/ml) of hair and Sepia melanin at neutral pH are shown in Figure 1. The absorption of hair melanin exhibits a structureless spectrum with absorptivity decreasing monotonically with decreasing wavelength. Sepia melanin exhibits a higher absorption at longer wavelengths, indicating that the particles are bigger than that of hair melanin. The longer wavelength absorption is attributed to scattering and absorption due to higher-molecular-weight melanin polymers. It is true that the spectral features of melanin change with size and that a sonicated suspension of hair melanin filtered through a micron-size filter displays an absorption spectrum with a decrease in longer wavelength absorptivity (data not shown).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)