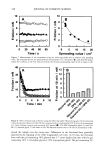
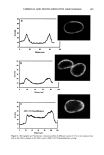
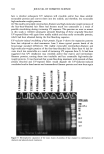
418 JOURNAL OF COSMETIC SCIENCE Log CFU• 7 0 5 10 15 Time (days) a I-7:1 Log CFU/g 7 o o 5 10 15 33 25 33 Time (days) Log CFU/g Log CFU/g '1 7- 0 5 10 15 2) 25 3D 0 5 10 15 2) 25 3D Time (days) Time (days) c d Figure 7. Survival curve of fungi in the sample with preservative (P) and without preservative (NP). (a) C. a/bicam, sample P. (b) C. a/bicam, sample NP. (c) A. ,iger, sample P. (d) A. ,iger, sample NP. One exception was noted in the curves for A..iger (Figure 7) that presented similar behavior for both treatments. These data indicate that the preservative in the powder was not effective against this organism. The same behavior can be noticed in Table II. The D-value average and the analysis of variance could not be calculated because of the low correlation coefficients. The results obtained against S. aureus in both samples (with and without preservative) indicated that the antimicrobial activity was effective according to the US and the Japanese pharmacopoeia requirements (6,9). However compared to the British and Eu- ropean pharmacopoeias (7,8) and the CTFA (10) requirements, just the preserved sample
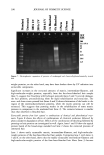
PRESERVATIVE EFFICACY TESTING METHOD 419 Table IV Regression Analysis for All Tested Microorganisms Using Sample With Preservative (P) and Without Preservative (NP) (y = a + bx) Microorganism a p (a) t (a) b p (b) t (b) R (%) S. aureus (P) 6.98 0.000 73.71 (0.082) 0.000 (20.94) 96.95 S. aureus (NP) 6.96 0.000 70.10 (0.005) 0.000 (16.48) 91.60 P. aeruginosa (P) 6.64 0.000 45.06 (0.072) 0.000 (11.82) 91.13 P. aeruginosa (NP) 5.98 0.000 31.71 (0.002) 0.000 (9.48) 84.85 C. albicans (P) 6.43 0.000 26.43 (0.191) 0.000 (6.55) 80.01 C. albicans (NP) 7.07 0.000 39.28 (0.025) 0.000 (7.89) 78.04 A. niger (P) 6.88 0.000 117.75 (0.002) 0.000 (10.36) 79.81 A. niger (NP) 7.02 0.000 153.88 (0.000) 0.775 (0.29) 4.47 was effective. Homogeneous results were again observed with P. aeruginosa and C. albicans. The samples with or without preservative were approved by all tests according to these specifications. When the sample with preservative was challenged with A. niger, the tests were accepted by the US and Japanese pharmacopoeias (6,9) and not approved by the other compendia previously mentioned. The sample without preservative did not pass the criteria of the British and European pharmacopoeias and the CTFA (7,8,10). All the results discussed above indicated that the tests for each kind of sample, for each kind of microorganism, presented similar behavior. Table IV shows a regression using all the tests for each kind of sample (P and NP), and also for each microorganism. The t values for "a" and "b" indicated that good models, describing the regression, were obtained. Added to that, one can observe different values of "a" and "b," mainly "b," between the P and NP samples. These results are in agreement with the ones shown in Figures 6 and 7. CONCLUSIONS It can be concluded that it is possible to use freeze-dried microorganisms as the inocu- lum in efficacy preservative tests for solid cosmetics. Added to that, all the microor- ganisms tested can be used to inoculate the sample during the first six months, once they maintain viability between 105 and 106 UFC/vial and their resistance. REFERENCES (1) C. W. Bruch, Cosmetics: Sterility vs microbial control, Am. Perfum. Cosmet., 86, 45-50 (1971). (2) J. Dony, Probl•mes microbiologiques pos•s par les cosm•tiques. J. Pharm. Belg., 30, 223-238 (1975). (3) J. L. Smith, Preserving cosmetics, Cosmet. Toiletr., 96, 39•41 (1981). (4) D. S. Orth and C. M. Lutes, Adaptation of bacteria to cosmetic preservatives, Cosmet. Toiletr., 100, 59-57, 63-64 (1985). (5) S. V. W. Sutton, M. A. Magee, and D. K. Brannan, Cosmetic Microbiology, D. K. Brannen, Ed. (CRC Press, Boca Raton, FL, 1997), pp. 95-126. (6) United States Pharmacopoeia, 24th ed. (United States Pharmacopeial Convention, Rockville, MD, 2000). (7) British Pharmacopoeia (Her Majesty's Stationery Office, London, 1999). (8) European Pharmacopoeia, 3rd ed. (suppl.) (Maisonneuve, Paris, 1999). (9) Japanese Pharmacopeia, 13th ed. (Society of Japanese Pharmacopeia, Tokyo, 1996).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)