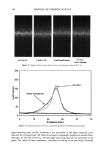
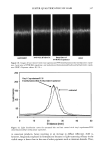
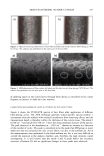
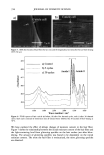
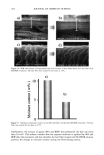
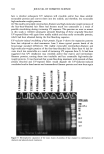
CHEMICAL AND PHOTO-OXIDATIVE HAIR DAMAGE 383 crosslink density in the matrix and form highly hydrophilic sulfonic acid groups (3). This leads to an increase in swelling and increased dye diffusion rates. Therefore, the higher the diffusion rate of a molecule into the hair fiber, the greater the changes in fiber morphology, that is, the greater the damage to the fiber. (a) Mapping of uranine diffusion in bleached hair, Our earlier studies involving chemical oxidation of hair fibers with hydrogen peroxide or a bleach cream containing ammonium persulfate had shown significant increases in the diffusion rates of uranine into the hair shaft, which is indicative of severe chemical and structural modifications resulting from the oxidative processes (2). In the present work, we have extended this method of using the diffusion of uranine as a measure of characterizing and quantifying morphological damage to photo-oxidatively degraded hair fibers. (b) Mapping diffusion of uranine in photochemically oxidized hair. Uranine is an anionic fluorochrome, which produces a green fluorescence in a basic environment and a yel- lowish-green fluorescence in an acidic solution. The fluorescence emission spectrum of diffused uranine in a dyed/unaltered hair fiber is shown in Figure 1. From the emission spectrum, the wavelength of maximum fluorescence of uranine is established at •m = 540 nm, and the instrument is calibrated at that wavelength to carry out the profiling of the diffused uranine in the hair fiber cross section. Representative fluorescence emission profiles of diffused uranine in untreated hair and hair fibers exposed to increasing cycles of UV radiation/humidification, accompanied by the corresponding micrographs, are shown in Figure 2a-c. It can be clearly seen that in the untreated fibers dyed under identical conditions to those of hair fibers exposed to UV radiation/humidification cycling, diffusion of the dye molecule is restricted to the cuticula (Figure 2a). However, in the photo-oxidized samples, increased diffusion of the dyestuff into the fiber interior occurs with increased exposure time (Figure 2b,c). FI(%) 100 - 8O 6O 4O 2O o 360 460 ß liB'" ' ' i ..... I' ' ! ,560 660 760 WAVELENGTH (nm ) Figure 1. Typical fluorescence emission spectrum of uranine in an untreated hair tiber.
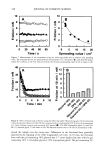
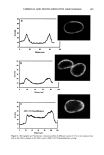
384 JOURNAL OF COSMETIC SCIENCE Calculating diffusion coefficients of uranine in untreated andphoto-oxidized hair. The diffusion profiles are used to calculate the diffusion rates of uranine into photo-oxidatively dam- aged hair fibers. From the diffusion profiles, the fluorescence intensities, which are proportional to concentration, were sequentially read at normalized distances "r" from the center of the fiber of radius "a." The fluorescence intensity at the "saturated" fiber edge is read as well. Using the concentration ratios C/C o for a specific, normalized distance "r" into the fiber with the radius "a," and for the fiber edge, an estimated diffusion coefficient was obtained from curves drawn by Carslaw and Jaeger (4) for Dt/a 2 by plotting C/C o vs r/a. This estimated diffusion coefficient from the Carslaw and Jaeger curves is then incor- porated into an equation to calculate the more accurate diffusion coefficients of the dye molecule into the hair shaft. The equation used is a modification of the Crank (5) equation for cylindrical systems, using the roots of the Bessel functions of the first kind of the order zero (1). C ø=(-Dt)Jo(r•,/a) -1-2Zex p •5-'[3, 2 ß (1) The diffusion coefficient is calculated from equation (1) by iteration. The calculated data are listed in Table I, which show significant changes in the diffusion coefficients of uranine in human hair fibers as a result of photochemical oxidation. For comparison, diffusion coefficients of chemically bleached (peroxide bleach cream) hair fibers from our earlier work (2) are included. The data in Table I are displayed in a graph in Figure 3. The graph and the table clearly show the increase in diffusion coefficients with the increase in the intensity of chemical and photo-oxidative processes. Clearly, the bleach cream containing ammonium persul- fate is the most effective oxidative process, that is, it causes the greatest modification of the morphology of the hair fiber, resulting in the greatest increase in the dye diffusion coefficient. This is indicative of the highest level of oxidative damage. The four-hour peroxide treatment does not show any significant increase in diffusion rate compared to the shorter, one-hour, bleaching time. Apparently, most of the morphological change to Table I Diffusion Coefficients of Uranine in Untreated and Modified Hair Fibers Hair sample D (m2/s x 10 •5) 0 h peroxide 3.59 + 0.67 1 h peroxide 9.78 + 3.67 4 h peroxide 10.30 _+ 0.87 0.5 h bleach cream 45.21 _+ 28.66 0 h UV 4.34 + 2.66 200 h UV/humidification cycling 6.47 _+ 5.44 300 h UV/humidification cycling 8.62 + 7.95 500 h UV/humidification cycling 18.18 _+ 6.72 600 h UV/humidification cycling (right side) 23.16 _+ 12.78 600 h UV cycling (left side) 20.39 _+ 7.76 Sample size: -10 hair fibers/category and -5 diffusion coefficients/hair fiber.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)