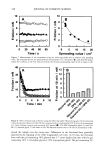
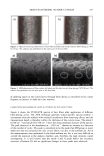
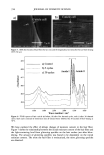
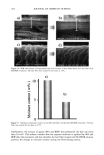
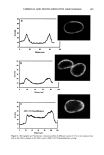
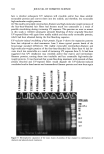
AMMONIA/PEROXIDE BLEACHING OF HAIR 407 j' . 500' nw, Figure 9. TEM image of hair melanosomes after 15 min of treatment with NH3/H202 at pH 10.0 (x40,000). A suspension of 0.05 mg/ml of hair melanosomes was treated with 1.0% ammonia and 1.0% hydrogen peroxide at pH 10.0. somes and provide new insights into the role of ammonia in the bleaching process. Aqueous ammonia treatment results in an initial breakdown of melanosome aggregates by removal of the surrounding amorphous material. This is followed by rupture of the melanosomal membrane sac, releasing melanin nanoparticles (Figure 7). The observation of melanin nanoparticles after ammonia treatment suggests that the micron-size hair melanosomes are comprised of 30-50-nm-size particles. In addition, these studies also provide evidence that, unlike Sepia, hair melanin particles are encapsulated in a mem- brane sac. Prolonged treatment of hair melanosomes with aqueous ammonia induced a complete destruction of characteristic melanosome morphology and a shrinking of the granule size, resulting in an amorphous material (Figure 8). By contrast, Sepia melano- somes under identical conditions of ammonia treatment did not show such changes. This might be due to differences such as metal content and the nature and type of metals present or differences in surface properties and aggregation behavior. Other alkalizing agents such as sodium hydroxide or sodium carbonate at pH 10 did not induce changes to melanosomes similar to those from aqueous ammonia. Thus, it is clear that ammonia-
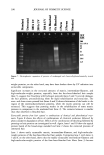
408 JOURNAL OF COSMETIC SCIENCE induced morphological and structural changes to hair melanosomes are not merely due to alkaline pH, but, rather, specific to ammonia. Hydrogen peroxide at neutral pH is found to be less efficient in bleaching hair mela- nosomes. However, TEM images of hair melanosomes treated with NH 3 and H202 revealed a complete distortion of the melanosomal structure immediately after treat- ment. The overall dissolution process is faster compared to independent treatments with either ammonia or peroxide. After l 5 min of bleaching, the nanoparticles released from the melanosomes had undergone a complete dissolution, and at the end of 30 min, a clear solution devoid of any particles was obtained. We believe that ammonia as a neutral species can diffuse across the melanosomal membrane and that, by a still unidentified mechanism, facilitate the rupture of the melanosomal membrane. Possible ways include an increase in intra-melanosomal osmotic pressure and/or inducing membrane protein conformational changes. TEM data clearly suggest that ammonia helps to release mela- nin nanoparticles from the melanosomes, making them more susceptible to oxidative attack by H20 2. This effect of ammonia does not preclude the likelihood that ammonia may mobilize or chelate transition metal ions present in hair melanosomes, facilitating the peroxide-induced bleaching. CONCLUSION In summary, the TEM data presented here provide direct evidence for the structural and morphological changes undergone by isolated melanosomes as a result of ammonia and ammonia/peroxide treatments. Our study clearly shows that ammonia has a specific role in the bleaching process, unlike other alkalizing agents, and that it facilitates the peroxide-induced bleaching of melanosomes. ACKNOWLEDGMENTS The authors thank Dr. Manoj Misra (AIM Unit) for providing the TEM facility, assis- tance in TEM measurements, and many helpful suggestions and comments towards the success of this work. They thank Ms. Karen Hoyberg for the ESEM studies. The authors also thank Dr. Jesse Weissman for many fruitful discussions. REFERENCES (1) L. Novellino, A. Napolitano, and G. Prota. Biochim. Biophys. Acta, 1475, 295-306 (2000). (2) C. R. Robbins, in Chemical and PhysicM Behavior of Human Hair, 3rd ed. (Springer Verlag, New York, 1994), pp. 131-152. (3) S. Ito, Biochim. Biophys. Acta, 883, 155-161 (1986). (4) S. Ito and K. Wakamatsu, Pig. Cell. Res., 11, 120-126 (1988). (5) G. Prota, Melanins and Melanogenesis (Academic Press, San Diego, 1992). (6) A.M. Kolb, E.G. Lentjes, N. P. Smit, A. Schothorst, B. J. Vermeer, and S. Pavel, Anal. Biochem., 252, 293-298 (1997). (7) J. Cheng,, S. Moss, M. Eisner, and P. Zshack, P(g. Cell. Res., 7, 255-262 (1994). (8) A. Napolitano, A. Pezzella, G. Prota, Rapid Comm. Mass Spectrum, 10, 468-472 (1996). (9) A. Napolitano, A. Pezzella, G. Prota, R. Seraglia, and P. Traldi, Rapid Comm. Mass Spectrum, 11, 368-372 (1997). (10) C. Kroesche and M. Pete, Tetrahedron, 52, 3947-3952 (1996).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)