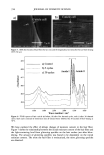
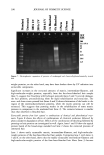
412 JOURNAL OF COSMETIC SCIENCE lation method of non-aqueous eye products such as loose and pressed powders consists of spraying on or adding microbial suspensions to the cosmetic, followed by mixing. Pressed eye shadows can also be tested by swabbing or spreading an inoculum on the product surface (10-12). There has also been developed a method to evaluate the surface of pressed powders in this method the test organisms on membrane filters are placed in direct contact with the products. The inoculation of microbial suspensions in powders described by the CTFA (10) is a procedure similar to the official ones for pharmaceuticals and cosmetics. However, it is difficult to guarantee homogeneity when mixing a microorganism suspension with a powder, since the volume of the inoculum must be minimal (not more than 1% of the total sample amount). The use of solid inoculum could facilitate the homogeneity between the microorganism and the sample. In this study a solid inoculum was obtained by using the process of freeze-drying. The efficacy preservative testing period is too long, since it lasts 28 days. There is an alternative method that has been used (13,14) that involves the microorganism death curve and the determination of the decimal reduction time (D-value), which is the time required for the reduction of 90% of the microorganisms. This curve can be constructed by determining the number of surviving microorganisms after 2, 4, and 24 hours for bacteria, and 4, 8, and 24 hours for fungi. The aim of this study was to evaluate the use of freeze-dried microorganisms to inoculate eye-shadow samples in preservative efficacy testing. In order to reach this goal, the results obtained were compared to the specifications in all the official compendia men- tioned above, to those of the CTFA, and also to the determination of the D-value. EXPERIMENTAL TEST ORGANISMS The test organisms were Staphylococcus aureus ATCC 6538, Pseudomonas aeruginosa ATCC 9027, Candida albicans ATCC 10231, and Aspergillus niger ATCC 16 404. STABILITY EVALUATION OF FREEZE-DRIED MICROORGANISMS Microbial suspensions were obtained as described in the US Pharmacopoeia 24 (6). Sus- pension drops of the inocula were used in order to have about 10 7 CFU/vial. They were transferred to five vials, and 1.0 ml of Molico © skim milk (Nestle) with 5% inositol (DIFCO) was added. The samples were frozen at -70øC, and the freeze-drying process began at -55øC using a Supermodulyo 12K © (Edwards) freeze-dryer. The vials were closed with rubber stoppers. The total aerobic count was determined by the plate count technique, using the five vials (6). These determinations were made immediately and one week, 15 days, and one, two, four, five, and six months after the freeze-drying process. MICROORGANISM RESISTANCE EVALUATION BY MINIMUM INHIBITORY CONCENTRATION (MIC) The preservatives used were methylparaben, propylparaben, and a combination of DMDM hydantoin and iodopropinyl butyl carbamate (Glydant plus©). The determina- tion was made by using the broth dilution technique in a 96-well microtiter plate (15).
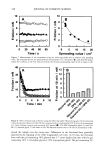
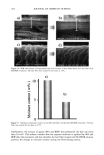
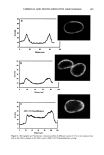
PRESERVATIVE EFFICACY TESTING METHOD 413 CHALLENGE TEST (10) The sample used was a powdered eye shadow with (P) and without (NP) preservative. The amount of 15 g of the sample was added gradually to the inocula (one-vial content of freeze-dried mass) and mixed after each addition. At least six tests were performed for each microorganism, and also for each kind of sample. The bioburden was determined by the plate count technique, according to the CTFA method (10). The number of colony-forming units (CFU)/g was determined immediately at 1, 2, 7, 14, 21, and 28 days after inoculation. DETERMINATION OF D-VALUE The procedure of inoculation was the same as that described above. The number of CFU/g was determined after 2, 4, 24, and 48 hours for bacteria and after 4, 8, 24, and 48 hours for fungi. The death curve was constructed, and the D-value was calculated by taking the negative reciprocal of the slope of the line obtained by linear regression of the plot of the log number of surviving microorganisms as a function of time after inocu- lation into the test sample. RESULTS AND DISCUSSION Loss of viability occurred after the freeze-drying process for all tested microorganisms except C. albicans (Figure 1). During six-month storage of the vials in the refrigerator, at around 5øC, oscillations occurred. Besides that, the amount of microorganisms remained at about 10 6 to 10 7 CFU/vial, the suitable load to use in the preservative efficacy test (6-10). Freeze-dried inocula have been especially developed to contaminate products such as oils (16). Although some modifications, such as the lack of plasmids (10,17), can occur after the lyophilization process, this process is still the best one to maintain microorganism 10- S. aureus P. aeruginosa C. a/b/cans A. niger Figure 1. Freeze-dried microorganism viability during six months. d: days. m: months.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)