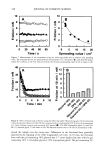
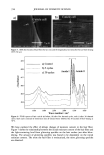
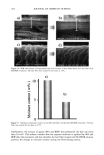
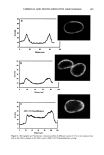
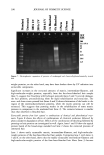
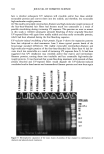
CHEMICAL AND PHOTO-OXIDATIVE HAIR DAMAGE 393 into easily soluble molecules. However, post-bleaching was not able to undo the UV- radiation-induced crosslinking and/or fusion of the proteins and degrade them into readily soluble/extractable molecules (lane 6). Hair fibers exposed to a combination of short-term bleaching/perming show high amounts of extractable matrix, intermediate-filament, and high-molecular-weight pro- teins (lane 8). However, subsequent UV exposure results in a considerable decrease in extractable intermediate-filament and high-molecular-weight proteins (lane 7), suggest- ing UV radiation-induced crosslinking and/or fusion of the originally easily soluble/ extractable proteins, which had been formed by bleaching/perming. Again, this seems to point to the possibility that UV exposure forms insoluble, and therefore less extractable, possibly high-molecular-weight, proteins by crosslinking. Embrittlement of the fiber supports this observation. Earlier in-house SEM studies had shown that after severe DTT extraction of both the intermediate filament and matrix proteins, a completely collapsed, tube-like shell of the hair fiber remained, seemingly consisting mainly of the cuticular sheath. Under the conditions used in this electrophoretic study, no specific information is obtained about the cuticular proteins of this residual cuticular shell. It may be speculated that the proteins of the A-layer and exocuticle are resistant to extraction under the conditions used in this study. Prolonged extraction may be necessary to achieve their extraction. On the other hand, proteins of the weakly crosslinked endocuticular domains of the cuticle cell break down easily and are more extractable. However, their molecular weights may be comparable to those of the matrix proteins and, therefore, indistinguishable from the latter in the extracted materials. CONCLUSIONS The microfluorometric study has shown that chemical and photochemical oxidative pro- cesses severely damage hair proteins and, as a result, significantly alter fiber morphology. Modifications of fiber morphology due to oxidative processes are quantifiable by mi- crofluorometry. There appears to be an initial resistance to photo-oxidative degradation. Short-term UV exposure appears to cause low levels of photodegradation, restricted to the peripheral region of the hair fiber. Comparing photochemical with chemical oxida- tion, short-term UV exposure results in slightly less damage than observed in hair fibers exposed to one and four hours of bleaching with hydrogen peroxide. However, long-term UV exposure results in more extensive oxidative damage than produced by chemical oxidation with hydrogen peroxide. The bleaching process involving the bleach cream containing ammonium persulfate, on the other hand, is more severe (the more effective oxidative process) than both photo-oxidative degradation and bleaching with alkaline hydrogen peroxide. Electrophoretic separation techniques can be used to establish the damage done to hair proteins by chemical and photochemical treatments as well as combinations of both. This study suggests that UV irradiation and perming may crosslink and/or fuse matrix and intermediate-filament proteins, turning them into insoluble and less extractable high-molecular-weight proteins. Long-term bleaching with peroxide and the use of bleaching/perming combinations, on the other hand, appear to degrade the matrix,
394 JOURNAL OF COSMETIC SCIENCE intermediate-filament, and high-molecular-weight proteins, preconditioning them for accelerated solubilization and extraction. These analytical methods are useful to quantitatively establish the level of morphological and chemical changes in the hair fiber caused by chemical and photochemical treatments as well as combinations of both. Such information will be helpful in selecting sunscreens, which are effective in preventing or retarding such damage. REFERENCES (1) R. A. F. Moore and H.-D. Weigmann, Characterizing fiber structure from dye diffusion behavior, Text. Chem. Color., 19, 13-18 (1987). (2) M. L. Tare, Y. K. Kamath, S. B. Ruetsch, and H.-D. Weigmann, Quantification and prevention of hair damage, J. Cosmet. Sci., 44, 347-371 (1993). (3) C. R. Robbins, Chemical and Physical Behavior of Human Hair, 3rd ed. (Springer-Verlag, New York, 1994), pp. 199-200. (4) H. S. Carslaw and J. C. Jaeger, Conduction of Heat in Solids (Clarendon Press, Oxford, 1947), p. 175. (5) J. Crank, The Mathematics of Diffusion (Clarendon Press, Oxford, 1956), pp. 66-67. (6) S. B. Ruetsch, Y. K. Kamath, and H.-D. Weigmann, Photodegradation of human hair,.]. Cosmet. Sci., 51, 103-125 (2000). (7) R. Kon, A. Nakamura, N. Hirabayashi, and K. Takeuchi, Analysis of the damaged components of permed hair using biochemical technique,.]. Cosmet. Sci., 49, 13-22 (1998).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)