J. Cosmet. Sci., 60, 475–484 (September/October 2009) 475 Infl uence of an extract from kudzu symbiosomes containing leghemoglobin on in vitro cutaneous procollagen production JAMES V. GRUBER and ROBERT HOLTZ, Arch Personal Care, 70 Tyler Place, South Plainfi eld, NJ 07882, and Bioinnovation Laboratory, 7220 W. Jefferson Ave., Lakewood, CO 80235. Accepted for publication June 15, 2009. Synopsis Cytoglobin is a hexacoordinate globin protein that was recently discovered in mammals. Interestingly, of the four human globin proteins that are now known, hemoglobin, myoglobin, neuroglobin and cytoglobin, the latter appears to have the closest resemblance to strikingly similar proteins expressed in plants. In legumes, these proteins accumulate in symbiosomes (root nodules) of various legumes and are called leghemoglobin. The paper will discuss the ability of an aqueous extract from Pueraria lobata (kudzu) symbiosomes that con- tains leghemoglobin to stimulate procollagen production in human dermal fi broblasts. This effect may be partly due to the possibility that leghemoglobin may mimic the function of cytoglobin by shuttling oxygen to prolyl-4-hydroxylase, the enzyme responsible for oxidizing proline residues in procollagen bundles. This hypothesis is supported by DNA microarray sequencing data that demonstrate that treatment of normal hu- man dermal fi broblasts (NHDF) with highly purifi ed cytoglobin or leghemoglobin upregulates a number of key collagen-related genes including COL1A1 and COL1A2. INTRODUCTION The recent discovery of a fourth globin protein in humans, called cytoglobin, which ap- pears to be expressed in human dermal fi broblasts and other cells that express collagen (such as osteoblasts), has raised the distinct possibility that these heme-based proteins may infl uence biochemical cascades in growing skin (1–3). Cytoglobin has been identi- fi ed within many tissue types in the body although its expression in skin cell fi broblasts is likely, but still not fi rmly confi rmed (4,5). The protein appears to be highly conserved in animal species (6,7). The possibility that expression of cytoglobin in skin cells that express collagen and other extracellular matrix fi brous proteins, namely fi broblasts, can be upregulated by hypoxia (oxygen-defi cient growing conditions) has been discussed, indicating that cytoglobin either plays a role in oxygen perfusion or acts as a oxygen sen- sor or perhaps both (4,8–10). Cytoglobin also appears to have important antioxidant properties that may play a role in cellular defenses (3,11–13). Recent work has demon- strated that cytoglobin can enter the nuclear envelope and infl uence the genome, directly raising the possibility that these proteins can infl uence cellular functions at the genetic level (14).
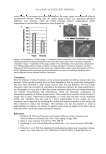
JOURNAL OF COSMETIC SCIENCE 476 Because cytoglobin appears to infl uence oxygen’s effects at the cellular levels, it has been suggested that cytoglobin may infl uence procollagen production by moving oxygen to key enzymes responsible for the early synthetic steps in collagen production (4,5,15). In particular, an enzyme called prolyl-4-hydroxylase, which is also a heme-based enzyme, requires oxygen to be attached at its active site in order to facilitate conversion of proline to hydroxyproline in early-stage procollagen production (Figure 1) (16). This paper will explore the possibility that plant-based substitutes for human globin proteins, namely leghemoglobin, may function in a similar capacity to assist procollagen production. All green plants express heme-based globin proteins, which are thought to function as free-radical scavengers and oxygen-transport molecules, much as they do in humans (17). While all green plants express globin proteins (called phytoglobins), cer- tain plants, namely legumes, which are known to fi x nitrogen, accumulate this protein in special structures found on the plant roots called symbisomes (generally known as root nodules). Pueraria lobata, a plant more commonly known as kudzu, is a legume that grows in many parts of the world. It grows rapidly and can extensively cover many other plants, which causes problem in countries where the plant has no real predators. The roots of kudzu contain symbisomes, and these can be easily harvested and extracted to provide useful quantities of leghemoglobin. The ability of plant-based hemoglobins to scavenge reactive oxygen species has been established (18). The paper will examine whether a plant-based extract containing leghemoglobin can infl uence the genes responsible for upregulation of extracellular matrix proteins in normal human dermal fi broblasts compa- rably to human-derived cytoglobin. MATERIALS AND METHODS PUERARIA LOBATA (KUDZU) SYMBIOSOME EXTRACT AND PUERARIN Using previously published methods, an aqueous extract taken from root nodules isolated from Puerraria lobata (kudzu) symbiosomes was standardized to 0.2–0.5 mg/ml leghemoglobin (3). Purifi ed puerarin was obtained from Sigma Chemical Company (Milwaukee, WI). Figure 1. The proposed role of cytoglobin to act as an oxygen shuttle to deliver oxygen to prolyl-4-hydrox- ylase, which, in turn, oxidizes proline residues to hydroxyproline residues in early-stage procollagen (steps a–b). The collagen fi gure was adapted from reference 16.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)