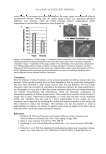
DETERMINATION OF RETINOIDS IN COSMETICS 491 determined by using a four-point calibration curve, with the concentration range used depending on the expected concentration of analyte. All peak areas were evaluated for accuracy of integration and manually reintegrated if necessary. RESULTS AND DISCUSSION OPTIMIZATION OF THE HPLC METHOD In most samples, each analyte’s peak was baseline separated and could be quantifi ed un- ambiguously. This separation was achieved using gradient elution with a mobile phase that was initially more polar (25% ammonium acetate buffer and 75% methanol) to re- solve the highly polar analyte, retinoic acid. The mobile phase was gradually changed to a less polar solvent (80% methanol and 20% dichloromethane) to elute retinol, which has an intermediate polarity. Finally, the mobile phase was gradually changed to an elutant with even lower polarity (70% methanol and 30% dichloromethane) to elute the ex- tremely lipophilic analyte, retinyl palmitate. During optimization of the mobile phase composition, the aqueous buffer concentration and pH were found to be important for ensuring good resolution of retinoic acid and to minimize peak broadening due to ioniza- tion of the retinoic acid. However, retinol is unstable if the pH is too low. An acetic acid/ ammonium acetate buffer at pH 5.5 was found to provide optimum separations. IDENTIFICATION OF RETINOIDS Retinoids were identifi ed by comparison of peak retention times and UV spectra with known standards. Figure 2A shows the chromatographic separation and elution order of standard retinoids, 13-cis-retinoic acid, 9-cis-retinoic acid, all-trans-retinoic acid, 13-cis- retinol, all-trans-retinol, all-trans-retinaldehyde, and all-trans-retinyl palmitate, whose re- tention times were 17.6, 18.5, 19.1, 20.5, 20.7, 22.0, and 38.9 minutes, respectively. As expected, the retention time was found to be inversely correlated with the polarity and correlated with the lipophilicity of the retinoid. Although available information suggests that all-trans-retinyladehyde is not used in cosmetics, it is included in the chromatography standard mixture to demonstrate the ability of the chromatographic system to resolve this retinoid from retinoic acid, retinol, and retinyl palmitate. All-trans-retinylaldehyde ap- pears as a negative chromatographic peak (Figure 2A) due to selection of 360 nm as a reference wavelength. Figures 2B and 2C show typical chromatographic separations of retinoids in extracts of cosmetic products containing either all-trans-retinol or all-trans- retinyl palmitate. Figures 2D and 2D show the chromatographic separation of retinoids in an extract from a lotion containing all-trans-retinol. The additional peaks seen in Figure 2D may be attributable to other isomers or oxidation products of retinoids in the lotion. Tests with standard 9-cis- and 13-cis-retinoic acid and 13-cis-retinol showed that the HPLC method could distinguish between these cis isomers and the respective trans iso- mers. Most products containing all-trans-retinol were also found to contain 11-cis-, 9-cis-, or 13-cis-retinol from less than 10 μg/g to 347 μg/g. Levels of all-trans-retinaldehyde from less than 10 μg/g to 138 μg/g were also observed in some products (data not shown). The levels of these components may refl ect differing amounts of 11-cis-, 9-cis-, 13-cis- retinol, and all-trans-retinaldehyde impurities in the all-trans-retinol raw material and/or
JOURNAL OF COSMETIC SCIENCE 492 Figure 2. (A) Chromatographic separation and elution order for standard retinoids 13-cis-retinoic acid (13-cis-RA), 9-cis-retinoic acid (9-cis-RA), all-trans-retinoic acid (all-trans-RA), 13-cis-retinol (13-cis-RL), all-trans-retinol (all-trans-RL), all-trans-retinaldehyde (all-trans-RAL), and all-trans-retinyl palmitate (all-trans-RP) (note: the negative peak for all-trans-retinaldehyde is due to the use of 360 nm as reference wavelength). (B) Chromatographic separation of extracted cosmetic cream containing all-trans-retinol with no evidence of other isomers or oxidation products. (C) Chromatographic separation of extracted cosmetic liquid makeup containing all-trans-retinyl palmitate with no evidence of other isomers. (D) Chromatographic separation of extracted cosmetic lotion containing all-trans-retinol with evidence of other isomers and/or oxidation products (note additional, unidentifi ed peaks). (D ) Expansion of D.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)