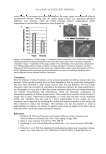
DETERMINATION OF RETINOIDS IN COSMETICS 493 varying rates of isomerization and oxidation of all-trans-retinol due to different product formulations, packaging, storage conditions, and the length of time the product was stored prior to sale. Other mono-cis isomers (e.g., 7-cis-) and di-cis isomers (e.g., 7,9-cis-, 9,11-cis-, 9,13-cis- and 11,13-cis-) were not studied due to their reported instability and probable low concentrations. Although chromatographic separation (peak-to-peak) of most retinoids was between 0.5 and 1.0 minutes or more, the peak-to-peak separation of 13-cis- and all-trans-retinol was only 0.2 minutes. It was necessary to confi rm the identity of these analytes using the HP ChemstationTM software purity factor based on their UV spectra. In addition, two other retinol isomers, 11-cis-retinol and 9-cis-retinol, for which standards were not commer- cially available, were identifi ed based on their UV absorbance spectra and λ-max in com- parison to published reference data (18). Concentrations for these two isomers were determined, based on their peak area and the calibration curve for the all-trans isomer. Retention times for 11-cis-retinol and 9-cis-retinol were 19.7 and 20.2 minutes, respec- tively. Since cis retinoid isomers are known to generally have lower extinction coeffi cients than the corresponding trans isomers (22), levels reported for 11-cis-retinol and 9-cis- retinol that are calculated using the extinction coeffi cients for the trans isomers must be considered overestimates or upper bounds for their concentrations. Retinyl palmitate isomers, other than the all-trans isomer, remain unidentifi ed since neither standards nor UV absorbance and λ-max data are available for these isomers. Figure 3A shows the UV spectra for standard retinoids 13-cis-retinoic acid, 9-cis-retinoic acid, all-trans-retinoic acid, 13-cis-retinol, all-trans-retinol, all-trans-retinaldehyde, and all-trans-retinyl palmitate. With the exception of all-trans-retinaldehyde, all of the reti- noids have UV absorption maxima at or near 330 nm. The UV spectra of the chromato- graphic peaks identifi ed as all-trans-retinol (Figure 3B), extracted from a cosmetic cream sample, and all-trans-retinyl palmitate (Figure 3C), extracted from a liquid makeup sam- ple, were consistent with UV spectra for the corresponding chromatographic standards. Figure 3D shows the UV spectra for the chromatographic peaks identifi ed in Figures 2D and 2D . UV spectra confi rmed the identifi cation of peaks in chromatographic analysis of the products’ extracts. RETINOID STABILITY Due to the instability of retinoids from oxidation and isomerization, antioxidants or other stabilizers must be included in cosmetic formulations containing retinol or other reti- noids to prevent decomposition thus BHT, EDTA, vitamin C, vitamin E, and/or other stabilizing agents were listed as ingredients in most of the samples analyzed (23,24). Many samples were also labeled with recommendations to seal tightly after opening. All products were opened and analyzed within one day. During sample preparation, ex- traction, and analysis, an effort was made to minimize exposure of samples to air, light, and heat. BHT was added to the extraction solvent to prevent oxidation during the ex- traction. Samples were found to be stable during sample preparation and extraction. This stability was confi rmed by the mass recovery results for all-trans-retinoic acid, all-trans- retinol, and all-trans-retinyl palmitate, and by the chromatographic results, which indi- cated no signifi cant isomerization, oxidation, or decomposition of all-trans-retinoic acid, all-trans-retinol, and all-trans-retinyl palmitate during extraction and HPLC analysis.
JOURNAL OF COSMETIC SCIENCE 494 Figure 3. (A) UV spectra for peaks in Figure 2A (standard retinoids 13-cis-retionic acid (13-cis-RA), 9-cis- retinoic acid (9-cis-RA), all-trans-retinoic acid (all-trans-RA), 13-cis-retinol (13-cis-RL), all-trans-retinol (all- trans-RL), all-trans-retinaldehyde (all-trans-RAL), and all-trans-retinyl palmitate (all-trans-RP) in the order of their elution). (B) UV spectrum for Figure 2B (extracted cosmetic cream containing all-trans-retinol with no evidence of other isomers or oxidation products). (C) UV spectrum for Figure 2C (extracted cosmetic liquid makeup containing all-trans-retinyl palmitate with no evidence of others isomers). (D) UV spectrum for peaks eluting in chromatographic analysis of an extract of a cosmetic lotion containing all-trans-retinol (see Figure 2D).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)