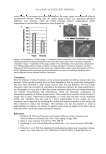
KUDZU EXTRACT AND PROCOLLAGEN PRODUCTION 481 Procollagen assay (Takara ELISA Kit). A series of Type 1 C-peptide standards was pre- pared, ranging from 40 ng/ml to 640 ng/ml. An ELISA microplate was prepared by re- moving any unneeded strips from the plate frame. In each well to be used 100 μl of peroxidase-labeled anti-procollagen Type 1 C-peptide was added, followed by 20 μl of either sample (one part collected tissue culture media diluted with four parts fresh cul- ture media) or standard. The microplate was then covered and allowed to incubate for 3 ± 0.25 hours at 37°C. After the incubation period, each well was aspirated and washed three times with 400 μl of wash buffer. After the last wash was removed, 100 μl of per- oxidase substrate solution (hydrogen peroxide with tetramethylbenzidine as a chroma- gen) was added to each well and the plate was incubated for 15 ± 5 minutes at room temperature. After the incubation, 100 μl of stop solution (1 N sulfuric acid) was added to each well and the plate was read using a microplate reader at 450 nm. Procollagen concentration calculations. A standard curve was generated using known concen- trations of Type 1 C-peptide. A regression analysis was then performed to establish the line that best fi t the data points. Mean absorbance values for the test materials and un- treated samples were then used to estimate the amount of Type 1 C-peptide present in each sample. RESULTS AND DISCUSSION HUMAN DERMAL FIBROBLAST MICROARRAY RESULTS Our interest in whether or not leghemoglobin, derived from symbiosomes of legumes, in particular symbiosomes of Pueraria lobata (kudzu), might behave like its human- derived counterpart, cytoglobin, was spurred principally by the fact that, like cytoglobin, leghemoglobin is a hexacoordinate heme-based globin protein. Using highly purifi ed samples of leghemoglobin and cytoglobin, we treated normal human dermal fi broblasts with the equivalent of 1% of each protein for 24 hours. The fi broblasts were then har- vested and the RNA was retrieved, fl uorescently labeled, and analyzed using a DNA microarray. It became immediately apparent when we examined the gene expression data for several important extracellular matrix proteins that, indeed, these two similar proteins could infl uence expression of collagen and elastin genes in very similar fashions (Table I). The apparent similarity between the fi brous protein genes upregulated by cy- toglobin and those upregulated by leghemoglobin is striking and suggests that, indeed, the very close similarity of these two proteins infl uences dermal fi broblasts in very simi- lar ways. PROCOLLAGEN ASSAY RESULTS ON HUMAN DERMAL FIBROBLASTS Examining the data from our initial studies in which we employed only the raw extract from kudzu symbiosomes, we noted that there appeared to be an increase in procollagen production attributable to the extract (Figure 2). Ascorbic acid was used as a positive control in these studies, as it has been shown in the literature to upregulate expression of Type 1A1 procollagen in human fi broblasts in vitro and in vivo (19). This initial assay appeared to indicate that the extract of kudzu symbiosomes did stimulate procollagen production in normal human dermal fi broblasts. However, it was immediately
JOURNAL OF COSMETIC SCIENCE 482 realized that Pueraria lobata extracts are known to contain also a glycosylated isofl avonoid called puerarin (Scheme 1). While studies on puerarin have not explicitly shown that this isofl avonoid can stimulate Type 1A1 procollagen production in dermal fi broblasts, it is certainly a possibility that such an effect could be occurring. TLC and HPLC analysis of the extract from kudzu symbiosomes demonstrated that it does contain puerarin at a level of approximately 0.4 wt%. Therefore, a defi nitive conclusion that the procollagen stimulatory effects were related to the presence of the leghemoglobin could not be made. The experiment was repeated, but two additional control samples were tested, purifi ed leghemoglobin and purifi ed puerarin. Data from the second study are shown in Figure 3. From the data available from the second study, it becomes immediately apparent that at the concentrations available in the crude extract, both puerarin and leghemoglobin pro- vide a dose-dependent stimulatory infl uence on Type 1A1 procollagen synthesis in nor- mal human dermal fi broblasts. However, it is apparent that, indeed, leghemoglobin can stimulate procollagen synthesis in support of published claims that cytoglobin, the structurally similar human protein, may play a role in oxygen transport during Figure 2. Type 1A1 procollagen analysis on normal human dermal fi broblasts with increasing concentra- tions of an extract of kudzu symbiosomes. Ascorbic acid was used as a positive control. Table I Gene Expression after Treatment of Human Dermal Fibroblasts with 1% Each of Leghemoglobin and Cytoglobin Fibroblast: 1% Leghemoglobin Name ID Ratio of medians (635/532) Gene name I_960109 A_23_P207521 1.9 COL1A1 I_930569 A_23_P255244 1.517 COL1A2 I_930697 A_23_P215459 1.459 ELN Fibroblast: 1% Cytoglobin Name ID Ratio of medians (635/532) Gene name I_960109 A_23_P207521 1.573 COL1A1 I_930569 A_23_P255244 1.55 COL1A2 I_930697 A_23_P215459 1.323 ELN Data shown is the ratio of medians for treated fi broblasts/untreated fi broblasts for each protein. Values of ratio of medians greater than 1.3 indicate a statistically signifi cant upregulation of the gene.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)