J. Cosmet. Sci., 60, 485–500 (September/October 2009) 485 Determination of retinol, retinyl palmitate, and retinoic acid in consumer cosmetic products JEAN C. HUBINGER, U.S. Food and Drug Administration, 5100 Paint Branch Parkway, College Park, MD. Accepted for publication March 26, 2009. Synopsis Retinol and retinyl palmitate are frequently used in cosmetic products. A simple, rapid, and sensitive re- versed-phase high-performance liquid chromatography (HPLC) method with ultraviolet (UV) detection was developed for the quantitation of retinol, retinyl palmitate, and retinoic acid in cosmetic preparations. The analytes were extracted from a cosmetic/Celite mixture using a solvent system composed of equal amounts of hexane, isopropanol, and ethyl acetate, and the extract was injected directly into an HPLC chromatograph with a C18 column and UV detector set at 330 nm. Chromatographic separation was achieved by gradient elution with a mobile phase, starting with aqueous ammonium acetate buffer/methanol that was gradually changed to methanol/dichloromethane. The average recoveries of retinol, retinyl palmitate, and retinoic acid from spiked cosmetic products were 95% or higher. In a survey of twenty-nine consumer cosmetic skin care products labeled to contain retinoids, most products were found to contain either retinol or retinyl palmitate at concentrations up to 2.2% (w/w), while a few products contained both ingredients. A number of products also contained cis isomers of retinol that could be quantitatively distinguished from the all-trans compound. The method can be used to quantitate several retinoids and their isomers in cosmetic products. The method will be useful for obtaining information needed to estimate levels of exposure to retinoids from cosmetic products. INTRODUCTION Retinol (vitamin A) is the parent compound of a large number of natural and synthetic compounds collectively referred to as retinoids. In addition to retinol, the primary bio- logically important and naturally occurring retinoids are retinyl palmitate, retinalde- hyde, and retinoic acid (Figure 1). These retinoids are essential for the development, growth, and health of vertebrates. One of the earliest biological functions discovered for retinoids was their critical role in the development and maintenance of healthy epithelial tissue, including the skin (1–3). Studies have demonstrated that the normal structure and function of the skin is dependent on the orchestration of cellular division, differentiation, and keratinization by retinoids (4). Discovery of the powerful effects elicited by retinoids in the skin has led to their wide- spread use in dermatologic drug products and cosmetics. Both topically and orally ad- ministered retinoids are currently used to treat dermatologic conditions such as acne and disorders of keratinization (e.g., psoriasis and ichthyosis) and to reduce the clinical signs
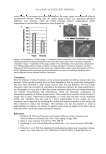
JOURNAL OF COSMETIC SCIENCE 486 of aging in the skin (5,6). Retinoids used in these dermatologic drug products are primar- ily isomers and synthetic analogues of retinoic acid. Commonly used active pharmaceuti- cal ingredients include all-trans-, 13-cis- and 9-cis-retinoic acid, commercially known as tretinoin, isotretinoin, and alitretinoin, respectively (Figure 1). Retinoids are also widely used in cosmetics. An indication of the frequency of use for retinoids in cosmetics may be obtained from the U.S. Food and Drug Administration’s Voluntary Cosmetic Registra- tion Program (VCRP). In 2008, approximately 30,000 cosmetic products were registered in the VCRP. Registered formulations included products containing retinol (160 prod- ucts), retinyl acetate (28 products), retinyl palmitate (1778 products), and retinoic acid (three products). Since participation in the VCRP is voluntary, these data may underesti- mate the frequency of use of retinoids in cosmetics. Retinoid-containing products regis- tered through the VCRP include moisturizers, skin cleaners, skin conditioners, lipstick, makeup foundations and bases, shampoos, and, increasingly, products marketed to reduce the appearance of aging and photoaging. The use of retinoids in this array of product categories increases the likelihood that a consumer may receive multiple daily exposures to retinoids due to the use of cosmetic products. Information on the levels of retinoids in cosmetic products is very limited. In 1987, the Cosmetic Ingredient Review (CIR) Expert Panel, an independent safety advisory group established in 1976 by the Cosmetic, Toiletry & Fragrance Association, reported that retinol and retinyl palmitate are generally used in cosmetics at concentrations ≤1% (7). A small number of products were reported to contain between 1% and 5% retinol (w/w) and between 5% and 10% retinyl palmitate (w/w) (7). The reported concentrations were Figure 1. Selected retinoid structures.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)