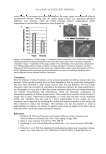
KUDZU EXTRACT AND PROCOLLAGEN PRODUCTION 483 procollagen synthesis. Both the DNA microarray data and the in vitro procollagen data suggest that normal human dermal fi broblasts cannot distinguish between topically ap- plied cytoglobin and topically applied plant-derived leghemoglobin in terms of the abil- ity of these molecules to infl uence procollagen synthesis. CONCLUSIONS It is tempting from the results of these experiments to conclude that globin proteins can infl uence procollagen production via control of oxygen transportation at the cellular level. However, such conclusions cannot be made directly from the data provided in these stud- ies. Because topical application of both cytoglobin and leghemoglobin appears to up- regulate the genes responsible for Type 1A1 procollagen synthesis, the effects seen here may be related to direct transcription infl uences, not to fundamental oxygen transport effects. Distinguishing whether cytoglobin (and indeed leghemoglobin) affect procollagen synthesis through oxygen control will be tricky, given that these proteins can infl uence Scheme 1. Chemical structure of puerarin. Figure 3. Type 1A1 procollagen results from kudzu symbiosome extract, ascorbic acid, and purifi ed leg- hemoglobin and purifi ed puerarin.
JOURNAL OF COSMETIC SCIENCE 484 these cells at the genomic level. Proof will likely come through more intimate studies done under conditions of hypoxia, where it is known that cellular cytoglobin upregula- tion has been demonstrated. However, even in these studies, separating genetic infl uences from oxygen-shuttling infl uences will remain fundamentally diffi cult. ACKNOWLEDGMENTS Samples of purifi ed human-derived cytoglobin and purifi ed plant-based leghemoglobin were kind gifts of Professor Mark Hargrove, Iowa State University, Ames, Iowa. REFERENCES (1) J. T. Trent and M. S. Hargrove, A ubiquitously expressed human hexacoordinate hemoglobin, J. Biol. Sci., 277, 19538–19545 (2002). (2) T. Burmester, B. Ebner, B. Weich, and T. Hankeln, Cytoglobin: A novel globin type unquitously ex- pressed in vertebrate tissues, Mol. Biol. Evol., 19, 416–421 (2002). (3) US Patent 20030198700A1. (4) M. Schmidt, F. Gerlach, A. Avis, T. Laufs, S. Wystub, J. C. Simpson, E. Nevo, S. Saaler-Reinhardt, S. Reuss, T. Hankeln, and T. Burmeser, Cytoglobin is a respiratory protein in connective tissue and neurons, which is upregulated by hypoxia, J. Biol. Chem., 279, 8063–8069 (2004). (5) K. Nakatani, H. Okuyama, Y. Shimahara, S. Saeki, D. Kim, Y. Nakajima, S. Seki, N. Kawada, and K. Yoshizato, Cytoglobin/STAP, its unique localization in splanchnic fi broblast-like cells and function in organ fi brogenesis, Lab. Invest., 84, 91–101 (2004). (6) A. Pesce, M. Bolognesi, A. Bocedi, P. Ascenzi, S. Dewilde, L. Moens, T. Hankeln, and T. Burmester, Neuro- globin and cytoglobin: Fresh blood for the vertebrate globin family, EMBO Reports, 3, 1146–1151 (2002). (7) D. Hamdane, L. Kiger, S. Dewilde, J. Uzan, T. Burmester, T. Hankeln, L. Moens, and M. C. Marden, Hyperthermal stability of neuroglobin and cytoglobin, FEBS J., 272, 2076–2085 (2005). (8) E. Fordel, E. Geuens, S. Dewilde, P. Rottiers, P. Carmeliet, J. Grooten, and L. Moens, Cytoglobin ex- pression is upregulated in all tissues upon hypoxia: An in vitro and in vivo study by quantitative real- time PCR, Biochem. Biophys. Res. Commun., 319, 342–348 (2004). (9) P. P. A. Mammen, J. M. Shelton, Q. Ye, S. B. Kanatous, A. J. McGrath, J. A. Richardson, and D. J. Garry, Cytoglobin is a stress-responsive hemoprotein expressed in the developing and adult brain, J. Histochem. Cytochem., 54, 1349–1361 (2006). (10) X. Guo, S. Philipson, and K. Tan-Un, Study of the hypoxia-dependent regulation of human CYGB gene, Biochem. Biophy. Res. Commun., 364, 145–150 (2007). (11) D. Li, X. Q. Chen, W. Li, Y. Yang, J. Wang, and A. C. H. Yu, Cytoglobin up-regulation by hydrogen peroxide plays a protective role in oxidative stress, Neurochem. Res., 32, 1375–1380 (2007). (12) E. Fordel, L. Thijs, L. Moens, and S. Dewilde, Neuroglobin and cytoglobin expression in mice: Evidence for a correlation with reactive oxygen species scavenging, FEBS J., 274, 1312–1317 (2007). (13) N. J. Hodges, N. Innocent, S. Dhanda, and M. Graham, Cellular protection from oxidative DNA dam- age by over-expression of the novel globin cytoglobin in vitro, Mutagenesis, 23, 293–298 (2008). (14) E. Geuens, I. Brouns, D. Flamez, S. Dewilde, J. Timmermans, and L. Moens. A globin in the nucleus! J. Biol. Chem., 278, 30417–30420. (15) A. Fago, C. Hundahl, H. Malte, and R. E. Weber, Functional properties of neuroglobin and cytoglobin. Insights into the ancestral physiological roles of globins, IUBMB Life, 56, 689–696 (2004). (16) M. M. Stevens and J. H. George, Exploring and engineering the cell surface interface, Science, 310, 1135–1138 (2005). (17) J. V. Gruber, Globin proteins: A fi rst line of defense against reactive oxygen species, Cosmet. Sci. Technol., 1, 69–75 (2005). (18) C. Seregelyes and D. Dudits, Phytoglobins and nitric oxide: New partners in an old signaling system in plants, Acta Biol. Hung., 54, 15–25 (2003). (19) P. K. Muller, E. Kirsch, V. Gauss-Muller, and T. Krieg. Some aspects of the modulation and regulation of collagen synthesis in vitro, Mol. Chem. Biochem., 28, 73–85 (1981).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)