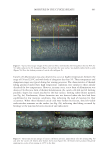
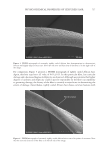
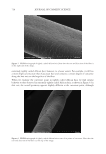
733 ENHANCED NATURAL OIL DEPOSITION shear-thinning profiles along with high clarity in a range of different natural triglyceride oils and their blends with other ingredients have been observed (6). The way oil deposits on the surface of hair can greatly impact the consumer perception in slipperiness, smoothness, and overall quality of the hair fibers (7). Quantification of natural oils is relatively straightforward using standard gas chromatography–mass spectrometry (GC–MS) techniques, but understanding the homogeneity of the oil deposition can be challenging to visualize, although it can have a profound impact on friction along the hair tress. In this study, we have demonstrated the utility of these acrylic cross-polymers and the improvements observed in coconut oil deposition, and we showcase visualization tools that further our understanding of the codeposition of acrylic copolymer with coconut oil. METHODS MATERIALS Methacrylic acid (MAA) was supplied by Dow Chemical (Collegeville, Pennsylvania, USA). Isobutyl methacrylate (IBMA), 2-ethylhexyl methacrylate (EHMA), and trimethylolpropane diallyl ether (TMPDE) were supplied by Sigma Aldrich (St. Louis, Missouri, USA). Three gram tresses of European hair, 8 hour bleached, 1 inch wide, was purchased from International Hair Importers (Glendale, New York, USA). Refined coconut oil was purchased from Sigma Aldrich. STRUCTURED OIL PREPARATION AND HAIR TRESS TREATMENT The acrylic copolymer used in this study was prepared using the process detailed in patent WO2020092032A1 with a composition of 79.5 IBMA/19.5 EHMA/1 MAA/0.10 TMPDE by weight. Latex emulsion was spray-dried and used as prepared. Spray-dried copolymers powders and coconut oil were heated to 50°C and mixed with an overhead mixer at 500 rpm for 1 hour to prepare the structured coconut oil. The prewashed 8 hour bleached hair tresses were treated with coconut oil (as supplied) or structured coconut oil (2% acrylic copolymers in coconut oil), left to dry overnight, and then washed with shampoo the next day. Coconut oil retained on the hair was quantified by GC–MS after extraction in hexanes, derivatization, and comparison to calibration standards. The extracted coconut oil was converted to the methyl esters (KOH/MeOH) and quantified by GC–MS using calibration standards prepared from coconut oil following the same derivatization protocol. Natural triglyceride oils (e.g., coconut oil) or natural oil- containing blends Spray-dried powder Structured/thickened oil Use thickened oil as-is (conditioning hair oil) Or emulsify into rinse- off conditioner Oil deposition Benefits from oil (conditioning) Oil wasted Greasiness Figure 1. Mechanism for structuring natural oils with acrylic copolymers.
734 JOURNAL OF COSMETIC SCIENCE RINSE-OFF CONDITIONER FORMULATION The rinse-off conditioner formulation used for the studies is found in Table I. Deionized water was added to the mixing vessel and heated to 70°C. With moderate agitation, the hydroxyethyl cellulose was dispersed until fully dissolved. The temperature was decreased to 60°C, and cetearyl alcohol and PEG-100 stearate, glyceryl stearate, and oil or oil gel were added. The conditioner was mixed for 3 min, and then tetrasodium EDTA was added and mixed for 3 min. When the temperature was below 40°C, the phenoxyethanol and methylisothiazolinone was added. Additional water was added, and the final pH of all conditioners was approximately 5. SECONDARY ION MASS SPECTROMETRY IMAGING Organic depth profiling of deposited layers can be accomplished using secondary ion mass spectrometry (SIMS). This method utilizes an Ar cluster ion beam (gas cluster ion beam) to gently etch the surface followed by analysis with a Bi 3 + ion beam with sensitivity to the molecular and elemental composition of the top 2 nm. In this case delayed extraction was used to obtain high-resolution images during depth profiling. Data were collected using an IONTOF V SIMS (IONTOF, Münster, Germany) instrument with a primary analysis beam operating at 30 kV using bunched Bi 3 + ions in a 200 μm random raster with 256 × 256 pixels. Depth profiling was accomplished using a 2.5 kV Ar 1000 + ion beam in noninterlaced mode with 1 scan/1 sputter cycle across 500 μm square raster areas. Data were analyzed using Surface Lab 7.2.125200 (IONTOF, Münster, Germany). RESULTS The main component of coconut oil is the ester of lauric acid, a saturated, linear dodecyl (C12) hydrophobe. To target the greatest compatibility with coconut oil, which contains mostly short-chain fatty esters, we selected a polymer composition with 80% IBMA and 20% EHMA, stabilized with a small level of MAA and lightly cross-linked for maximum viscosity enhancement. The compatibility of these acrylic copolymers is highlighted in Table I Rinse-Off Conditioner Formulation Used for the Studies Control Coconut oil only 6% acrylic copolymer in coconut oil gel Ingredient (INCI name) wt% wt% wt% Water 95.8 93.8 93.8 Hydroxyethyl cellulose 1.5 1.5 1.5 Tetrasodium EDTA 0.2 0.2 0.2 Cetearyl alcohol 1.0 1.0 1.0 PEG-100 stearate and glyceryl stearate 1.0 1.0 1.0 Oil or oil gel 0.0 2.0 2.0 Phenoxyethanol and methylisothiazolinone 0.5 0.5 0.5 EDTA: ethylenediaminetetraacetic acid INCI: International Nomenclature Cosmetic Ingredients.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)