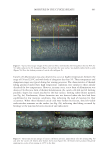
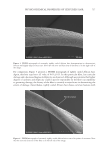
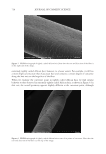
697 Address all correspondence to Jennifer M. Marsh, marsh.jm@pg.com UV Oxidation: Mechanistic Insights Using a Model System JENNIFER M. MARSH, STEPHANIE L. DAVIS, RUI FANG, MONIQUE S. J. SIMMONDS, PHILIP GROVES AND VICTOR CHECHIK The Procter & Gamble Company, Mason Business Center, Mason, Ohio, USA (J.M.M., S.L.D.) Royal Botanic Gardens, Kew, Richmond, Surrey, United Kingdom (R.F., M.S.J.S.) Department of Chemistry, University of York, Heslington, York, United Kingdom (P.G., V.C.) Synopsis Damage to hair by UV radiation is relevant to most people, and for many, it is a major source of hair damage. The chemistry is complex, and studying all of the detailed reactions is extremely challenging. The objective of this work was to create a model system that allows for the study of one key mechanism involved in UV damage: the oxidation of tyrosine. A colloidal system to study the reactivity and chemistry of tyrosine was tracked by monitoring tyrosine decomposition and dityrosine formation by fluorescence spectroscopy. Experiments showed both the important role of oxygen in the decomposition of tyrosine and how the addition of redox metals such as iron can accelerate this decomposition. Finally, an antioxidant, butylated hydroxytoluene, was demonstrated to reduce this oxidation through the interception of reactive oxygen species. These findings suggested two possible strategies for reducing UV-induced radical damage to hair: removal of redox metals and addition of a radical scavenger to react with reactive oxygen species formed. The second strategy was confirmed by testing selected extracts from tea (Camellia sinensis), which is well known to have antioxidant properties. An oxygen radical antioxidant capacity assay was used as a screening tool to identify which tea extracts have the highest antioxidant efficacy, and these extracts were shown to reduce UV-induced protein damage in hair. INTRODUCTION Healthy hair is described as hair that has shine, can be readily styled, and has low levels of frizz in high humidity. In contrast, damaged hair shows signs of breakage, lack of alignment, split ends, and frizz (1). This difference is driven by multiple insults including oxidative colorants, UV exposure, heat, and physical damage from grooming habits such as washing and combing. Exposure to too much sun leading to lighter hair and damaged tips is a well-recognized issue for women, and breakage is often observed after prolonged exposure. Degradation of different parts of the hair structure by UV radiation has been well-documented in the literature, with changes to cuticle and cortex protein structures (2), cell membrane lipids (3), and melanin (4). J. Cosmet. Sci., 72, 697–710 (November/December 2021)
698 JOURNAL OF COSMETIC SCIENCE The different chemical mechanisms causing these changes are complex and difficult to measure directly in hair. There is strong evidence that two key amino acids in hair that absorb UV-B radiation are tryptophan and tyrosine. These amino acids are photoionized and produce aromatic free radicals. Tryptophan oxidation produces yellow-colored kynurenines (5), which can be observed as a photo-yellowing effect in light-colored hair. Singlet oxygen and a superoxide radical anion are formed by the photosensitization of both aromatic amino acid residues and melanin pigments (6). Cell membrane complex unsaturated lipids such as the unsaturated fatty acids react with singlet oxygen to form hydroperoxides that modify the cell membrane complex and provide hydroxyl and alkoxyl radicals for additional reactions. These reactions have been studied and reported in both hair and wool (2,7). Reactive oxygen species (ROSs) that are formed continue to propagate damage throughout the hair. It has also been shown that redox metals such as copper can accelerate these radical reactions and create additional protein damage (8). An approach to reducing UV damage is to terminate the reactions by quenching free radicals or ROSs. Primary antioxidants, such as polyphenols, act in this manner. There are three main mechanisms by which antioxidants can scavenge ROSs: hydrogen-atom transfer, single-electron transfer, and metal chelation. Polyphenols can act as antioxidants by these mechanisms and are important because they are commonly found in botanical extracts including tea (Camellia sinensis) (9). Several publications have studied the use of tea extract for hair and skin benefits (10), including as protection against UV-induced skin damage and in the development of sunscreen products (11). Tea extracts have also been studied for their impact on hair growth (12) and sebum reduction (13). The objective of this study was to create a model system for the key components in hair involved in the initiation of radical pathways and gain a more detailed understanding of specific pathways involved, including the role of redox metals. From this work, the strategy of adding antioxidants was identified as an option for reducing oxidative damage. A selection of tea extracts that can prevent UV damage by intercepting the ROSs formed were screened and shown to reduce this oxidative damage. MATERIAL AND METHODS POLY(ETHYLENE GLYCOL)–TYROSINE BLOCK COPOLYMER MICELLES Poly(ethylene glycol)–tyrosine (PEG–Tyr) polymers (prepared according to published literature procedures [14]) were kindly donated by Prof. A. Heise, Dublin City University, Dublin, Ireland (14). PEG 5000 –Tyr 15 (20 mg, 0.00259 mmol), PEG 2000 –Tyr 5 (10 mg, 0.00344 mmol), and PEG 5000 –Tyr 10 (20 mg, 0.00293 mmol) were separately added to water (1 mL). The mixtures were sonicated at room temperature for 60 min to ensure homogenous dissolution into colloidal systems. The colloidal systems were irradiated using a 100 W mercury arc lamp (Oriel 6281/ Ushio USH-102DH) with spectral irradiance ranging from deep UV through infrared wavelengths. To add iron(III) stearate and iron(III) acetylacetonate to the polymer, stock Fe(III) solutions were prepared. Specific volumes of the stock solution were transferred into sample vials to give the desired metal concentration when diluted by a factor of 100. The ethanol was then removed under a vacuum, and PEG 2000 –Tyr 5 polymer solutions (0.5–5 mg/mL, 0.074– 0.735 mM) were prepared in 0.1 M pH 5 acetate buffer and added to dried Fe residue. The
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)