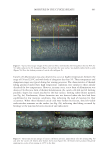
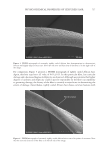
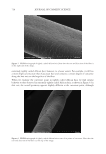
701 UV OXIDATION into glass scintillation vials. A 10 mg/mL solution of matrix-assisted laser desorption/ ionization (MALDI) matrix (α-cyano-4-hydroxycinnamic acid) was mixed with the hair extract samples in a 1:1 volume ratio. Then, 1 μL of this solution was used to spot on the MALDI plate, and a MALDI mass spectrum was acquired (1,000 shots). The intensity of the UV damage marker peptide at m/z 1,278 was measured. This biomarker was identified as a fragment of the S100A3 protein that is involved in cuticle cell adhesion (24) and is directly related to the level of UV exposure of untreated hair. CAMELLIA SINENSIS (TEA) EXTRACTS Commercially available C sinensis extracts and powders were obtained from seven suppliers of botanical ingredients. The materials were described by the suppliers as either green or white (nonfermented) tea extracts. The number of samples per supplier varied from one to three. Each material was assigned a unique five-digit botanical identification code (BIC). BICs are used to identify the materials throughout this article. Details about the chemistry of these extracts were published by Davis et al. (31). A summary of the findings are presented here to support the role of catechin compounds in reducing oxidative damage and the role of the ORAC assay in evaluating this activity. RESULTS AND DISCUSSION PHOTOCHEMISTRY MODEL SYSTEM The only amino acids capable of direct photoexcitation by sunlight are those with significant absorption above 280 nm. These are limited to those with aromatic side chains: tryptophan, tyrosine, phenylalanine, and histidine (although phenylalanine and histidine do not absorb significantly at 280 nm) (15). Tryptophan is the most effective UVB-absorbing chromophore among the aromatic amino acids, but given that tyrosine has far greater abundance than tryptophan in many keratin proteins (16) and hair overall (17), it is likely that tyrosine photochemistry is relevant to the overall photochemistry of hair proteins. In Scheme 1. Summary of the main reaction pathways of tyrosine upon photoexcitation (20–22).
702 JOURNAL OF COSMETIC SCIENCE aqueous solution at neutral pH, tyrosine exhibits two absorption bands at wavelengths of about 220 and 275 nm, which are assigned to π–π* transitions (18). Upon absorption of a photon, the amino acid is excited to the first singlet excited state. The subsequent pathways are shown in Scheme 1. Once formed, tyrosyl radicals can undergo a wide variety of reactions. Two radicals can combine through either a C–C linkage or a C–O linkage to form dityrosine (19) (Scheme 2). Initial experiments were performed on tyrosine itself, but its photochemistry was not amenable to study. In solution, any tyrosyl radicals formed were too short-lived to be studied, but as a solid, the radicals that formed persisted for many hours. These data highlighted the importance of the molecular environment for photochemistry pathways. This phenomenon has also been seen in hair studies in which protein degradation was found to be highly dependent on relative humidity (23). The aim in this work was to create a system with a restricted tyrosine environment that still allowed for some molecular mobility. Toward this end, a series of amphiphilic PEG– Tyr block copolymers were used (Figure 1) (14). The hydrophilicity of the PEG block and the hydrophobicity of the Tyr block cause the copolymers to self-assemble into kinetically trapped, stable colloidal structures upon dissolution in water. Upon heating, the solutions spontaneously convert into more thermodynamically stable gels (24). Fluorescence spectroscopy was used to monitor the loss of tyrosine and formation of dityrosine. Samples were irradiated in 1 cm-path-length quartz cuvettes. Initial experiments used the PEG 5000 –Tyr 10 polymer irradiated for 3 and 24 h, with fluorescence of the sample measured relative to that of a control sample prepared at the same time but kept in the Scheme 2. Formation of dityrosine by the combination of two tyrosyl radicals. Figure 1. Structure of PEG–Tyr block copolymers.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)