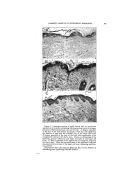
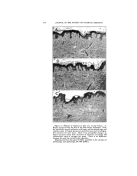
318 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS gross efficiency of the systems are not identical. The important fact to us is that in each instance, the best emulsion is obtained at about an HLB of 10. This is one of the major points on which this system is based. A given oil has an opti- mum HLB for a given type of emul- sification. Different oils require different values as ,nay be seen in Fig. 2. In The optimum required HLB val- ues indicated in Fig. 2 were for O/W emulsions. It will be noted that at tintes a W/O emulsion is formed. This usually appears at the extreme left-hand side of the figure. When this occurs, the oil is suitable for use in W/O formula- tions in major amounts. Often good emulsions are obtained of both types and the-oil can have an opti- mum HLB for either type of emulsion. When we speak of a required HLB, reference is usually made to the O/W type. ,,o0 Fortunately, the range covered by the inverse • . or W/O type appears to ..• be quite narrow and in -'.,• the low HLB's so that •o,.o the difference is readily apparent. •."-'- Required HLB values :T • for blends of oils •nay be calculated in a manner similar to that tbr blends of entulsifiers so long as the requirements for the components are known. Basis for this is illustrated in Fig. 3 in which all mineral oil is emulsified in the top row, 50/50 mineral oil-stearic acid in the middle row, and all stearic acid in the bot- tont row. The optimum emulsifier blend progresses front about 10 for 100% mineral oil to about 16 for 100% stearic acid. A natural question is the adher- ence of the HLB system to ionic or soap emulsions. Monovalent soaps, generally used for emulsification, ex- Figure 3.--Effect of blending oils and waxes this figure, one of the pairs of non- ionic emulsifiers used in Fig. 1, Span 40-Tween 40, is pictured with eight different oils or waxes cot- tonseed oil, lanolin, petrolatum, mineral oil, paraffin, cetyl alcohol, .beeswax, and stearic acid. It will be apparent that to choose an emul- sifter for a formula we need to know the required HLB for the oil blend, as well as that of the proposed emul- sifiers.
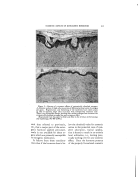
CLASSIFICATION OF SURFACE-ACTIVE AGENTS 319 . . Figure 4.--Behavior of soaps--varying ratios of oleic acid and NaOH hibit high HLB's, higher than re- quired for optimum emulsification. However, soap alone is seldom, if ever, the sole emulsifier in a for- mula. We know that soaps hydrolyze, and the true emulsifier is a mixture, or blend, of soap and free fatty acid. This is illustrated in Fig. 4: where the samples with the arrows are the theoretically neutralized soaps, but in each instance, the samples to the left in which there is excess fatty 'acid are the better emulsions. The emulsifier, so-called, in these series ranges from all fatty acid at the left to all base at the right. This might be considered as repre- senting increasing HLB from left to right, though it is only true to a point slightly to the right of the theoretical soap. At this point suf- ficient base has been added to mini- mize hydrolysis of the soap. Any higher ratio of base to fatty acid merely results in a reduction of the total amount of fatty acid and a re- duction of the amount of soap. Goodey (1) has studied the be- havior of emulsifiers and has ex- plained, in part, their action by de- fining a part of the emulsifier as a coupler. This is not coupling action as the word is generally used (the addition of an agent to promote clarity of solution of a concentrate or emulsion). He suggests that there are four constituents of an O/W emulsion: oil, water, emulsi- fier, and coupler. In his explana- tion, the emulsifier is always a highly hydrophilic substance and the coupler is always oil-soluble. The coupling action of which he speaks is promotion of solubility of the emulsifier in the oil phase to further the reduction of interfacial tension and improve emulsification. We prefer to consider all of this action under the term emulsification and to call both components emulsifiers.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)