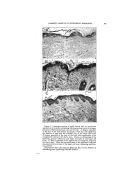
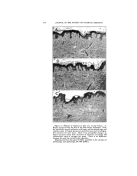
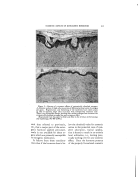
38O JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS of the essential oils were unstable contrary to those with aromatic chemicals. The life of the soap emulsions was shortened from two days to less than one hour. 3. The emulsions with Duponol were not affected by the essential oils under consideration. 4. The emulsions with Lecithin were somewhat less stable than the control. 5. The essential oils did not affect the Gum Arabic emulsions. study the ingredients of the perfume used especially when the liquid- type emulsion is made. There is an obstacle, however, in the practical application of this recommendation because the perfumes are often being supplied by outside concerns which naturally consider the composition of their products a trade secret. Consequently this point will involve much controversy. CONCLUSIONS DISCUSSION In common practice, the com- pound for perfuming the emulsions is always a mixture of 50-75 per cent aromatic chemicals and 25-50 per cent essential oils. However, some- times only one or a few aromatic chemicals or essential oils are used. Because of the variety of the char- acter of the perfuming ingredients, one ingredient may disturb the emulsion the other may tend to sta- bilize it with the result that emul- sion stability has not been affected. But this is a very rare coincidence on which the technician should not count. The experiments performed show that the matter is more com- plicated because of the great variety 1. It can be stated that in most of the experiments, both aromatic chemicals and essential oils had a varied effect on all five types of emulsions by often shortening but less frequently lengthening their life. 2. It seems that the rose char- acter of both aromatic chemicals and essential oils was destructive to the Triethanolamine emulsions. 3. Hydroxycitronellal is most de- structive to the three types of emul- sions. BIBLIOGRAPHY (1) Betkmart, S., and Egloff, G., "Emulsions and Foams," New York, Reinhold Pub- lishing Co. (1941), p. 58. (2) Corran, J. W., "Some Observations on a Typical Food Emulsion," in "Technical Aspects ooe Emulsions," London, A. Har- of the emulsifiers as well as of the in- vey, 1935, p. 91. gredients of the cosmetic emulsions. (3) Jannaway, S. P., "Toilet Preparations," .• Perfumery Essent. Oil Record (August, The safest recommendation is to 1939).
PENETRATION AND COMPLEX-FORMATION IN MONOLAYERS* By J. H. SCHULM^• and J. A. FR•E•r Department of Colloid Science, Cambridge University (England) IF ^ MONOLAYER OF an in- soluble substance is spread at an air-water interface, and a soluble substance containing a polar or ion- ized group attached to a non-polar structure (e.g., organic acids, soaps) is introduced into the underlying solution, in low concentrations (1 - 10 X 10 -6 g./cc.), there may be considerable changes in the surface pressure and potential of the film. If this is so, the soluble substance is said to penetrate the monolayer (1, 2). Be- cause of the chemical nature of the substances concerned, it is clear that there may be interaction between the polar head groups, or between the non-polar portions (van der Waals forces), or between both portions, and the nature of the phenomenon is determined by the relative importance of the two kinds of forces (3). In the first place, there may be strong polar interaction associated with weak van der Waals forces, as when benzoic acid is injected be- Dr. Schulman addressing the Society of Cosmetic Chemists of Great Britain, November 9, 1949. * Basis of an informal talk by Dr. Schul- man, presented at the Society of Cosmetic 'Chemists of Great Britain, Meeting, No- vember 9, 1949, London, England. neath a film of a long-chain amine (4). In this case a process of solu- tion of the soluble substance in the monolayer takes place, which may be reversed by compressing the film, 381
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)