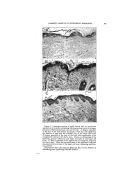
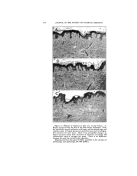
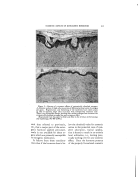
320 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ß ß Figure 5.--Comparison of fixed "coupler" content us. fixed HLB at different emulsion concentrations Goodey's data and ours agree that the agents involved must have the proper hydrophile-lipophile balance. lie suggests further that a proper hydrophile-lipophile balance for both the coupler and emulsifier is needed and that a given concentra- tion of coupler is needed in the oil phase to "attract" the hydrophilic emulsifier to the interface. There- fore, a 20% oil emulsion would con- tain twice as much coupler as a 10% oil emulsion. The emulsions in Fig. 5 indicate that this is not true. Rather, for a given oil and water there is one HLB for the total emulsifier that is best without regard to the concentra- tions of ingredients in the usual ranges. By way of illustration, the two pah's of series of samples con- tain various amounts of oil and water, the amount of oil decreasing TABLE 4•PERCENTAGE COMPOSITION OF EMULSION IN FIG. 5 Sample No. I • 3 4 5 Row 1 Oil 60 50 40 25 Water 34.3 45.0 55.8 71.9 Span 20 HLB 8.6 4.5 3.8 3.0 1.9 Tween 20 HLB 16.7 1.2 1.2 1.2 1.2 Row 2 Oil 60 50 40 25 Water 34.0 45.0 56.0 72.5 Span 20 HLB 8.6 4.5 3.8 3.0 1.9 Tween 20 HLB 16.7 1.5 1.2 1.0 0.6 Row 3 Oil 60 50 40 25 Water 34.5 45.0 55.5 71.2 Span 40 HLB 6.7 3.0 2.5 2.0 1.3 Tween 40 HLB 15.6 2.5 2.5 2.5 2.5 Row 4 Oil 60 50 40 25 Water 34.0 45.0 56.0 72.4 Span 40 HLB 6.7 3.0 2.5 2.0 1.3 Tween 40 HLB 15.6 3.0 2.5 2.0 1.3 10 88.05 0.75 1.2 10 89.00 0.75 0.25 10 87.0 0.5 2.5 10 89.0 0.5 0.5
CLASSIFICATION OF SURFACE-ACTIVE AGENTS 321 from left to right. The samples in the first and third rows were pre- pared with a fixed percentage of low HLB emulsifier in the oil phase to conform with Goodey's require- ments for a "coupler." In the sec- ond and fourth rows, all of the samples had the same ratio of low and high HLB emulsifiers. The per- centages are shown in Table 4. It is seen that a constant HLB for the total emulsifier provides a more uni-. form emulsification. In Table 5 we have listed several estimated HLB values for emulsi- fiers. These were determined and correlated by preparing vast series of emulsifier-blend tests as in the illus.trations. Grouping in this table is according to composition as well as HLB since both are influential in choosing an emulsifier. We hope to extend this list, and expect to do so. The magnitude of this type of study is understood when you real- ize that each of these values was derived from approximately 75 emulsions. A more extensive tab- ulation of estimated HLB values for Atlas products is available in the Atlas Surface Active Agents Booklet (6). We have found the chart, Fig. 8, most helpful for rapid calculation of HLB values of blends of emulsifiers. The location ofglyc- eryl monostearate in Table 5 is of interest. This reference is to the pure form and not to the self-emul- siftable varieties. The available self-emulsifiable types are not non- ionic, sir•ce ionic emulsifiers (soap, etc.) are added to render them self- emulsifiable. This is an example of a blend of non-ionic and ionic emul- sifters. These blends are satisfac- tory when no electrolytes antagonis- tic to the ionic portion of the blend are to be employed. In such cases a totally non-ionic emulsifier should be used. TABLE Emulsifier Esti- mated HLB Anionic T. E. A. Oleate xz Lecomene O •z.7* Sodium Oleate • 8 Potassium Oleate 20 Cationic Atlas G-zs• z5-35 Non-Ionic Oleic Acid App. I Span 85 t .8 Arlacel C 3- 7 Span 8o 4.3 Span 60' 4,7 Span 2o 8,6 Tween 8• •o.o Tween 6o i4. 9 Tween 8o •5.o Tween •o • 6.7 Other values listed in booklet, "Surface Active Agents," published by Atlas Powder Co. * Tentative value. The various types of emulsifiers represented in Table 5 may be blended, observing the usual pre- cautions of incompatibility. In ad- dition, there are indications that some emulsifiers are not compatible, or perhaps co-operative is a better word, in a surface-active sense. This action is probably related to over- all efB_ciency of the emulsifier and is therefore not conclusively indi- cated. Chemical reactivity of an emui- sifter can modify the behavior of an
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)