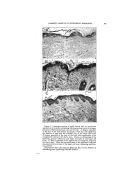
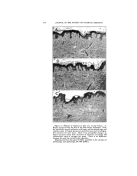
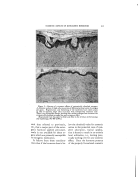
SURFACE-ACTIVE AGENTS IN COSMETICS* By H^RR¾ C. SPEEL tintara ]roducts, New York, N.Y. THE TASK OF CHOOSING the proper surface-active agent to use in formulating specific cosmetic prod- ucts is often a complex problem. Evidence of this is found in the numerous lists and attempts to classify the hundreds of such agents that are offered to formulatots in virtually every field of industrial activity (2, 4, 17, 23, 28). Actually, the total consumption of surface- active agents by cosmetic manufac- turers is relatively small, probably less than 5 per cent of the available production. But the existence of thousands of cosmetic manufac- turers with their millions of custo- mers makes the cosmetic field a po- tential customer for almost every new product the chemical manufac- turer can make. If the new agent is not quite suitable, he will try to "tailor-make" one more suitable, thus further increasing the available number. The term "surface-active agent" is, of course, a broad one applied to organic chemicals used for wetting, penetrating, emulsifying, dispersing, solubilizing, foaming, and deter- gency. Personally, I like the defi- nition of a surface-active agent being a product which brings unlike * Presented at the December 3, 1947, Meeting, New York City. surfaces together closer, faster. It imparts a sort of "sex appeal." a quality widely advertized for cer- tain soaps. Indeed, soap is the oldest and best known of all surface- active agents made by man. These agents are all characterized by their ability to modify the surface prop- erties of the medium to which they are added. For example, most agents will make water wetter, though certain types are superior as penetrating wetters and others excel as spreaders. One kind of agent will cause a liquid to foam another will kill foam. Emulsifying types help make oil and water mix and stay mixed. Dispersion of solids in a liquid is aided by certain agents other agents reverse the process. No one product excels in all applica- tions. The problem is to classify these agents and to match their peculiar properties with the needs of formulation of specific products. The primary functions of a sur- face-active agent are the result of proper blending, or balancing, of the raw materials from which it is made. Synthetic surface-active agents, like common soap and agents that occur in nature, are the result of a com- bination in one molecule of a water- seeking or hydrophilic portion, and an oil-seeking lipophilic, or hydro- 346
SURFACE-ACTIVE AGENTS IN COSMETICS 347 phobic portion. Molecules of this type orient themselves at the sur- face of a liquid, and also at the inter- face between two immiscible liquids. The water-seeking hydrophilic part stays in the water surface, while the oil-seeking hydrocarbon portion of the molecule stays out above the surface, as it were, or finds a non- aqueous medium, thus bringing un- like surfaces together closer, faster. The hydrophilic portion may be either ionic or non-ionic, as ex- emplified by carboxylate, sulfate, sulfonate, quaternary ammonium, polyhydroxylic and polyoxyethylene residues. The hydrophobic part consists of hydrocarbon chain or ring systems as are found in natural fats (26) and petroleum products (29). So many varied combinations are possible that products can be "tailor-made" to give whatever blend or balance of properties may be desired (1, 9, 27, 30). CLASSIFICATION The alphabetical listing of prod- ucts by brand name or trade name permits a start on more complicated methods of classification. Such lists (17, 25, 28) are continually being revised, as are alphabetical listings of manufacturers and their specialties (25). The next logical step is the alphabetical listing of those agents used, or advocated for use, in specific fields. Beeler's re- port to the American Pharmaceu- tical Association (2) and the bul- letins prepared by Cuppies of the U.S. Dept. of Agriculture's Bureau of Entomology and Plant Quaran- tine (4) are examples of this. An- other method of classifying is by chemical structure. Separation of anionic, cationic, and non-ionic types can be made in such a group- ing (23) as is given in Table 1, where R = long chain paraffinic or olefinic group--usually C8 to C== R' = primary or secondary, straight or branch- chain lower alkyl group --usually C4 to C•0 A = anion--C1, Br, I, HSO4 Ar = aryl group--phenyl, naph- thyl, diphenyl, etc. M = alkalimetal, ammonium or substituted ammonium, e.g.., Na, K, trierhanoi- amine. This example of chemical classi- fication is obviously only a starting point. It is certainly possible to subdivide cationics and non-ionics also on the basis of whether the hydrophilic group is in the center or at the end. Phosphonates and borates could go up in the anionic grouping instead of being put in miscellaneous. The sulfonium com- pounds could be added to the cat- ionic group. The fatty amide con- densates offer an unusual example of products difFtcult to classify they are compatible with soap and some anionic agents, as well as with a number of cationics they seem to be mildly anionic on the alkaline side and mildly cationic under acid con- ditions their chemical structure is a matter of debate. It is also possible to manufacture surface-active agents which contain cationic groupings as well as anionic groupings, e.g., the betaines (alkyl glycines). Never- theless, the grouping of surface-ac- tive agents by differences in chemi- cal structure is of value.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)