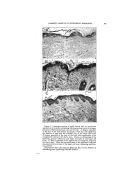
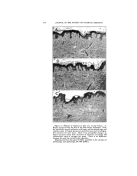
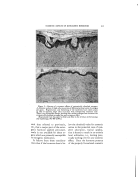
348 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS TABLE 1--CHEMICAL CLASSIFICATION' OF SURFACE-ACTIVE AGENTS Group Formula of Typical Representative Type Type Description A Anionic--Terminal Hydrophile Group RCOOM 1 Soap ROSOgOM 2 Fatty Alcohol Sulfate RCONHC•H4OSO2OM 3 Sulfated Hydroxye thyl Amide RCOOC•H4OSO•OM 4 Sulfated Fatty Acid Partial Ester RCH•OOCCH2SO2OM 5 Fatty Alcohol Sulfoacetate RArO(C•H,O),•C•H.•OSOgOM 6 Sulfated Polyglycol Ethers B Anionic--Central Hydrophile Group (R%CHOSOgOM 1 Sulfated Secondary Alcohol R'CHR' 2 Sulfonated Hydrocarbon I SODOM R:ArSO:OM 3 Alkyl Aryl Suloeonate R'OOCCH2CHCOOR • { Sulfonated Ester of Alcohols and I Dibasic Acids SO2OM C Cationic--Usually Terminal Hydrophile Group [RN(CHa)3]+A - 1 Long-Chain Quaternary Ammo- nium Salt [R-?yridine]+A- 2 Alkyl Tertiary Aryl Ammonit•m Salt [RNHa ] +A- 3 Long-Chain Amine Salts D Non-ionic RCOOCaH•(OH)2 1 Polyhydric Alcohol Partial Fatty Acid Ester RCOOCoH,O(OH)a 2 Anhydro Polyhydric Alcohol Par- tial Ester RCH•O(C•H40)nC•H•OH 3 Fatty Alcohol Polyoxyethylene Ether RCOOCoHaO [O(C•H40),,C2H4OH ]a { ?olyoxyalkylated Anhydro Poly- hydric Alcohol Fatty Acid Ester RArO(C2H•O),,C•H4OH 5 Alkyl Phenol Polyoxyethylene Ether Hydropho be-hydrophile Phos- phates, Phosphonates, Bo- rates, Fatty Amides, etc. E Miscellaneous A few generalities based on gen- eral experience are hard to avoid. For example, compounds derived from the lauryl hydrophobic radical are usually superior as wetting and penetrating.agents. The myristyl, cetyl, and stearyl hydrophobes con- stitute the best potential radicals for detergency and emulsification. Most commercially important, syn- thetic wetting agents and detergents are to be found in groups A and B of Table 1. But where heavy metal salts or complex cations are apt to be encountered, the tendency of an- ionics to form insoluble salts often makes them unsuitable, or less suit- able than the non-ionics of group D, for detergency or other operations. The agents of group C are attracted by negatively charged substances but repelled by positively charged substances, and are apt to be more stable than anionics in the presence
SURFACE-ACTIVE AGENTS IN COSMETICS 349 concentrated acids, alkalis, and saline solutions. So cationice find use in marine paints and varnishes, iii}i!jii:i:•:i•::iin carbonizing wool, in certain tex- ?:!.!!ftile softeners. Their' germicidal :::•:.'•:i)•:•':?applications are well known. The agents of group D tend to act as ?:::•:• '::spreaders, or surface wetters, and are widely employed as emulsifiers. Unfortunately for any hope of suc- :•)½}:•:?:cessful classification along these •?•:.: :::' lines, no one group has a very strong monopoly on any particular set of properties. In actual practice physical and :::•::• .::.chemical combinations of two or more of these agents are often used. Thus compatibility becomes an im- P. •'•:•}::::portant hctor•one readily found ¾•.: :•:•. on testing but one not evident in usual methods of classification. One example is the use of glyceryl mono- •)'i?:•:f. stearate, a non-ionic, in emulsion creams using soap formed in situ as the major emulsifier. Combinations :•: .':'.' of two different fatty amides, or two non-ionics are also well known to everyone. The textile field, with its :.::? ::softening oils, and the war-time program of oiling woolen blankets, has shown the value of utilizing anionic or cationic agents com- blned with non-ionics to give stable emulsions that are substantive to fabrics, animal hair, and inanimate objects carrying over of these techniques to cosmetic items is on the increase. COSMETIC PKOPEKTIE8 What properties, other than those shared in common by all surface- active agents, are important to cos- metic applications? Physical form, appearance, color, odor, sometimes even taste, and cost per pound cer- tainly deserve consideration. Dif- ferences in physical properties, such as foaming ability, or physical form, or chemical stability under con- ditions of usage are naturally very important factors. Most of these properties are obvious as soon as a sample of the product and technical data are made available to the cos- roetic chemist. The requirements of specific formulations therefore en- able him to pick out a score or more possibilities out of hundreds of agents available. Dermatological aspects are of pri- mary importance (20). The prod- uct should be safe when applied to the skin under possible condition of usage. Incidently this means that even toxicity on ingestion must be considered (3, 21, 22), for children and their pets may try to eat or drink the product. There is also evidence that wetting agents may bring about penetration of the skin, if not directly perhaps through emulsification and transfer to the glands (11). A recent study by Dodd, Hart- mann and Ward (6) on surface-ac- tive agents as potential irritants in ointment bases is worthy of note. Nine surface-active agents were tested for irritant properties on rabbits and human beings. In the series only the ionic surface-active agents were irritating to human skin, while the non-ionics were non- irritating. Their data seems to
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)