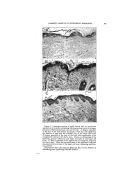
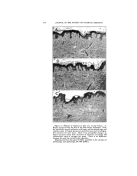
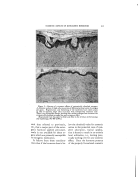
402 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS the procedure, to the effects of the component parts of the material used in the test media (agar or broth), to the nature of the test organism (unaffected or partially damaged), as well as to the varia- tions in the diffusion rates as brought about by the nature of the vehicle used for the testing (i.e., the solvent used for the medication). These tests include: 1. Dilution Methods ß (a) Using serial dilutions of the chemical in nutrient broth, inoculating the same with a heavy inoculum of a healthy growing culture of the test organism, incubating for 48 hours at 37øC., and observ- ing the end point, which will be where growth starts. That concentration used just prior to the one which permits growth would be considered to be the bacteriostatic strength. (b) A similar procedure wherein the serial dilutions and inocu- lations are made in nutrient agar. (c) A method similar to (b) ex- cept that the agar is not seeded, but, rather, is streaked across the surface with fresh viable organisms. Growth of organisms on the agar surface furnishes the end-point data in a similar manner to the above. 2. Zone-of-f nhibition Methods (a) This is the so-called Standard F.D.A. agar-cup or filter- paper procedure, as outlined in Circular 198 of theU. S. De- partment of Agriculture. (b) The writer's "Zone-Reduc- tion" method (details of test to follow). In previously published papers (5) the writer has shown that there may exist a fallacy in the standard zone test (2a) as far as the interpretations of its results are concerned. That is, namely, that the relative effi- ciency of a compound from a bac- teriostatic standpoint is not in pro- portion to zone size. In fact, it may even be inversely proportional. This we explain on the basis that it is due to the fact that the size of the clear area or zone produced by this test is determined, not by the 'factors which bring about the bacterio- static efficiency of the product but rather, for the most part at least, by the solution potential or diffusion power of the vehicle in which the medication has been incorporated. Thus, the same dilution of a specific chemical can be shown to have all the way from a trace to several millimeter zone size produced under identical conditions of test. For ex- ample: Tx•tz 1 Chemical Vehicle Base Zone Size, Min. Hexachlorophene (0•1%) Hexachlorophene Hexachlorophene " Petrola tum-lanolin GlyceryLmonos tearate Carbowax blend
DETERMINING BACTERIOSTATIC POTENCY OF CHEMICALS uOGEsTED SIMPLIFIED METHOD-- ONE-REDUCTION" METHOD the basis of the above assump- ibns we have developed a test which believe serves to give a reliably .•: •urate answer as to the bacterio- potency of a chemical, and more simple to perform than any the others mentioned above. It involves using the standard D.A. zone technique (filter-paper ) in reverse. Basically, :this new method, instead of g the zone size as produced bY'ithe chemical we determine the '•[miting concentration of the chem- ical at the point where no clear 7: •s produced. discussed above, various phys- i•al: and physico-chemical factors •?::may enter in to •nfluence the d•L • . . '•/•.• ::.:fus•on rate or soluuon pressure, as •?•::• •- . . . •?•:•:•mell as mutual solubd•ty relauon- •.•i:•sh•ps, which factors will determine •'how far the chemical "M" will diL •:•j•5•'fuse •nto the agar m the t•me T, :•5•::•'Which is that time such as is re- •*•3•:•:•quired by the bacteria being tested •.:•*• •: to overcome its lag-phase influences •?and start growing again. Thus, the :•'•5•:•:Point where these two opposing •?'forces meet will set the size of the ?•'•.clear zone area. However, contrary to what some others claim, it is the :.w •ter s opinion that the zone size ß •.•? •s not a criterion of the relative ?•'/': bacteriostatic e•ciency of the prod- )• •::•: uct. '•:•':"•::• Following through on this same •):?.•':basis it seems to us that as long as ? :there is any "M" available in the •..•:.: agar it can and will contact a "B." •::::: So long as such a contact is possible, 403 or does actually occur, we have bacteriostasis available or in ac• tion. Therefore, in order to deter- mine the real efficiency of the prod- uct, all that we need to do is to ap- ply the material absorbed into the filter paper in successive serial dilu- tions onto the surface of seede• agar and then incubate it in the normal manner. Then, at the end of the selected time period (24 hours, 48 hours, or 72 hours, etc.) remove the filter papers from the agar surfaces and examine under a microscope the area where the papers previously rested. About 25 magnifications is sufficient. Where .no bacteria exist in this area, which will always be the case if any "clear zone" is produced, one has no difficulty oeo so observe it. When some organisms are growing in this area, say up to 50 per cent of those present in the agar outside of the filter-paper area, it is still quite easy to make such an ob- servation without question. This situation continues to apply up to about where a 25 per cent reduction in colony count is present. From there on, to determine the exact end- point where a zero reduction oc- curs (thus representing no action between M and B) may be difficult. However, the difference in dilutions or concentrations of M as required to produce a 25 per cent reduction in colony count compared to that as required to show zero reduction does not appear to be materially sig- nificant, or enough to make it a serious factor. Equally great er- rors, on the percentage basis, occur
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)