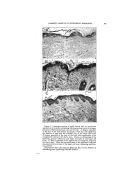
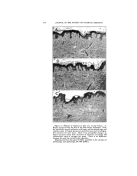
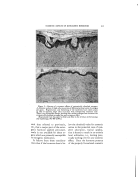
THE EFFECT OF SOME AROMATIC CHEMICALS AND ESSENTIAL OILS UPON THE STABILITY OF COSMETIC EMULSIONS* By S. A. Kauas, Sc.D. Bronxville 8, N.Y. IN VIlE COt•USE OV several years' experience in manufacturing emulsions, it was found that the liquid emulsions of the O/W type behaved irregularly regardless of the rigid control of all ingredients except perfume. This effect could not be explained otherwise than to blame the perfume. It is generally known that perfume is quantitatively the smallest item in every cosmetic formula, its amount usually being 1 per cent or even expressed in the form q. s. (quantity sufficient) and is given last in the formula. Since the importance of a good cosmetic emulsion is evident, even the small- est item of the formula should not be overlooked in order to obtain satisfactory results. In recent years many new in- gredients have been presented to the cosmetic industry with technically elaborate data. However this data, as helpful as it ig in improving the manufacture of emulsions, does not cons!der the entire specific task. For instance, none of this helpful data considers the effect of per- * Pre•ented at the May 20, 1949, Meeting, New York City. fume upon the stability of emul- sions. In emulsion literature, no one, to the author's knowledge, has pointed out sufficiently the. action of perfume upon the stability of emulsions. In dealing with food emulsions, Cotran (2) stated that the flavoring of mayonnaise does not exert any effect upon the per- sistence of emulsions. Berkman and Egloff (1), however, pointed out that the stability of an emulsion is determined by the coincidental action of various factors, such as the type of dispersion, temperature, pH values, viscosity, preservation, electrolytes, etc. Jannaway (3), writing on the stability of cosmetic emulsions, stated more specifically that all of th• constituents of an emulsion should be carefully con- sidered. After this clear statement by a cosmetic specialist, one would expect to find some reference to per- fume. Furthermore, the same au- thor, in writing on the perfuming of toilet preparations, considers many aspects of good cosmetic emulsions but not their stability in regard to perfume. He 'and others stressed the irritation of the skin by per- 374
:i:i•?i.•fume, the discc loration of cos- •?'irnetics, their preservation, the last- .:..,:.:: ing quality of perfume, and other Ji'v: subjects but there is no mention •:":".:: of the action of perfume on the sta- i!7)i 57 bility of emulsions. Let us now consider the effect of ' ?::!..5 that last but not least important in- 517..:' gredient in the formula, perfume. It 555:/was noticed that the emulsions were ¾.:-,•.. superior when the perfume ingredi- i: '":':: :::•,:: ents were controlled with care. By 5711ji::changing the ingredients in com- i:i:•i i pounding the perfume or by having them supplied by an outside firm, the emulsions were thus often ren- :::::i:: dered unsatisfactory. In view of " this fact, several experiments were :5. performed which are outlined in the following discussion. Five different types of emulsions were made, and the effects upon them of 1 1 aromatic chemicals and 10 essential oils were studied. " In the following discussion, the word "separation" is synonymous with the word "breaking." SUMMARY OF EXPERIMENTS To make the emulsions, the in- gredients were those most commonly used in the cosmetic industry. The emulsion was the liquid O/W type made with surface-active agents and also two emulsifiers of natural ori- gin, all favoring O/W emulsions. These emulsifiers were as follows: 1. Triethanolamine Stearate 2. Castile soap, powdered 3. Duponol ME (sodium lauryl sulfate) 4. Lecithin soybeans (phospho- lipid) 5. Gum Arabic (pentosan colloid) EFFECT OF CHEMICALS ON STABILITY OF EMULSIONS 375 The oils used were mineral and sesame. The aromatic chemicals and essential oils listed below were added separately to the five above emulsions. In so doing, 115 experi- ments were performed (21 perfum- ing ingredients added separately to each of the five emulsions thus re- suiting in 105 experiments and 10 controls not perfumed). In the dispersion of the oils, laboratory high speed Epenbach colloidal mills were used. Atromatic Chemicals 1. Phenylethyl Alcohol 2. Hydroxycitronellal 3. Terpineol 4. Benzyl Acetate 5. Linalyl Acetate 6. Geraniol 7. Linalool 8. Benzyl Alcohol 9. Methyl Ionone 10. Amylcinnamic Aldehyde 11. Methyl Anthranilate 12. Control not perfumed Essential Oils 1. Geranium 2. Bergamot 3. Lavender 4. Orange (sweet) 5. Patchouly 6. Vetivert (Bourbon) 7. Sandalwood 8. Neroli (Bigarade) 9. Rose de Mai 10. Ylang Ylang 11. Control not perfumed Experiment _r--Emulsions with Triethanolamine 50 gm. Triethanolamine 150 gm. Stearic Acid (triple- pressed) 3000 cc. Distilled Water
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)