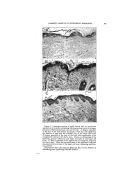
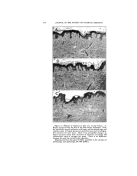
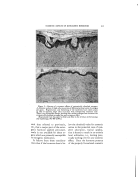
332 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS In this connection the problem arises as to whether a detergent solution is capable of cleaning out the skin pores and, if so, to what depth and how rapidly. It is a widespread and understandable fal- lacy to suppose that those deter- gents which show the highest wet- ting power by a Drayes test or a Seyferth-Morgan test must neces- sarily penetrate the skin pores most •ffectively. This is not the case. The canals of the skin glands form a typical capillary system. Wash- burn's equation for the rate of pene- tration of a liquid into a capillary is: dl (rG cos 8) dt 4l v where r is the radius of the capillary, G is the surface tension, 0 is the contact angle, 1 is the distance in the capillary, and P' is the viscosity. When 0 is zero the rate of penetra- tion is proportional to the surface tension/viscosity ratio. When 0 is between zero and 90 ø , high surface tension actually promotes penetra- tion. This implies that detergents will aid the penetration of a capillary mass only where the contact angle at the capillary walls must be re- duced to below 90 ø . Practically any detergent will reduce the con- tact angle to below 90 ø , and the best penetration should therefore be attained by agents of r•elatively high surface tension and low viscos- ity. This effect has actually been checked in the case of textile fab- rics. It is noteworthy that wetting and spreading, as measured by the spreading coefficient, S, depends on a zero contact angle and very low values of surface and interfacial tensions. S = Gb-- G•-- Gab where the Gs are the surface ten- sions of the substrate b, the spread- ing liquid a, and the interfacial ten- sion between a and b. A liquid which would be ideal for wetting and spreading over the stratum comeurn would accordingly be slow to penetrate the pores, and vice Another consideration of great importance in skin cleansing is the adsorption of the detergent itself on the skin. This problem is inti- mately connected with the subjec- tive sensation or "feel" associated with the detergent, and the objec- tive symptoms of irritation such as might be observed by a dermatolo- gist. It is well known that certain synthetic detergents cause com- plaints of dryness. The writer is unaware of any quantitative meas- ure of the dryness of skin, but the explanation is often advanced that these particular synthetic deter- gents "defat" the skin. This expla- nation is at best incomplete. All de- tergents including soap, under nor- real conditions of use, will remove sebum thoroughly. So will rub- bing alcohol, which tends to make the skin feel moist, and look shiny rather than dry. Certainly none of the detergents can remove fatty matter from the interior of the cells in either the stratum comeurn or the lower strata of the skin. A more comprehensive hypothesis of the drying action of detergents takes
COSMETIC FUNCTIONS OF SYNTHETIC DETERGENTS 333 into account the deposition of an adsorbed layer of the detergent on the skin. It has been proved that most detergents leave an adsorbed layer of their own molecules on tex- tile fibers after having removed all the original soil. Soap is known to leave a layer of fatty acids and acid soaps on textile fibers and also on the skin. These layers may have a variety of effects. If no layer is left and the pore linings are not injured as in the case of rubbing alcohol, the se- bum soon builds up and the skin feels normal. A layer of free fatty acid deposited from neutral soap, or of glyceride oil deposited from sul- fated oils, feels normal and pleasant to most individuals and apparently does not disturb normal functioning of the skin. The adsorbed layer of a "drying" detergent may possibly exert a tanning action on the kera- tinized outer cells, or it may ad- versely influence the living cells in the pore linings, thus diminishing the flow of sebum. The adsorbed layer may also, by its chemical nature, evoke the sensation of "dry- ness" in the nerve endings without objectively disturbing the skin func- tions. These are speculations, and have as yet no positive claim to va- lidity. They are not, however, con- tradicted by any known facts con- cerning the interaction of detergent and substrate in a detersire system. They are supported by the fact that the various detergents differ mark- edly in their drying effect on skin and this drying effect bears no re- lationship to their efficiency as oily soil removers, at least as measured on fabrics. The writer is unaware of any quantitative tests on the power of various detergents to re- move sebum from skin. Some very poor detergents such as the lower alkyl naphthalene sulfonates feel extremely drying on the skin, whereas some excellent detergents such as the alkyl aryl ether sulfates leave the skin feeling bland and pleasant. The above considerations hold true at comparable pH values, and although pH is known to have a marked effect on cutaneous reac- tions it is not the decisive factor in this case. With regard to shampoos the above picture is somewhat further complicated. The scalp and hair normally carty a higher soil load than the skin and the mechanical difficulties of cleansing are therefore magnified. The semidetached, ker- atinous, dead cells tend to accumu- late on the scalp in the form of dandruff, and these must be re- moved. Any adsorbed film left on the hair must be lustrous and non- tacky and should contribute to rather than detract from the soft- ness and manageability of the hair. Drying effect on the scalp is fully as important as it is in the case of skin cleansers. The possibility and effect of adsorbed films on both hair and scalp must accordingly be consid- ered. It should be noted that a de- tergent which leaves a lustrous, clean, soft hair shaft might never- theless leave a tight dry feeling on the scalp and thus be unsatisfactory to the user.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)