THE COLOR OF RED HAIR 779 During the past 20 years, other investigators have extracted similar pigments from red chicken feathers (9, 10). These results suggested that red iron pigments were also present. More recently, Barnicot (11), ex- tracted from human red hair a yellow solution with weak alkali at room temperature. Upon boiling in weak acids for five minutes, this solution turned red and assumed the characteristic absorption spectrum of tri- chosiderin. Barnicot's finding has provided further proof that tri- chosiderin is not an artifact of keratin hydrolysis. The progress of the past 20 years in the isolation and purification of tissue components persuaded us to resume the study of this unique sub- stance. It was hoped that the pigment of red chicken feathers would prove sufficiently similar to trichosiderin to serve as an experimental model, thus overcoming the difficulty of obtaining sufficient quantities of red hair. RECENT RESEARCH ON RED HAIR PIGMENT The first aim of the work, the isolation of various forms of iron pig- ments from chicken feathers has been achieved (12). All these forms have absorption bands at about 280 mt•. This paper describes the over- all direction and some general aspects of these studies. In all major respects the pigment of red chicken feathers behaved as its human counterpart. It had the same indicator properties in its "siderin" form, precipitated at pH 7, had the same absorption spectrum, contained iron, and could be degraded to derivatives analogous to those of trichosiderin. This finding is of considerable biologic importance. It suggests not only that the iron pigment is phylogenetically very old, but also sheds new light on the relative importance of trichosiderin. Considerably larger amounts of pigment could be extracted from feathers than from hair. Boiling for ten minutes with 0.1N HC1 yields 6-10 times more pigment than two hours of boiling of hair. This finding was not surprising, because on a macroscopic and molecular level feathers are looser structures than hair and would release their pigment more easily. However, feathers of Rhode Island Red chickens are not notice- ably redder than bright red hair. It appears that a large proportion of trichosiderin may be retained by the compact hair shafts. Conse- quently, during the prolonged extraction the pigment may be attacked by the extracting hot acid and made insoluble in the hair shaft. This hypothesis was tested by prolonging the extraction of hair and feathers with boiling 0.1N HC1 for several hours. Although the solu- tions obtained in the later stages are pale yellow or virtually colorless,
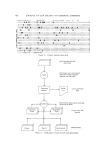
78O JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ,oo • •.,• I sf hour ""' - •' ,?_nd hour 8o '•L'. .............. .3rd hour 40 '\ '". 20 '• '"" I • 200 300 400 500 600 /• in rn)4 Fig. 1. Absorption spectra of trichosiderins in 0.1N HC1 with increasing length of extraction from hair. At arrow the concentration of the solutions was decreased to obtain readings at shorter wavelength upon neutralization small amounts of pigment may be precipitated from them. These late precipitates are increasingly brown their absorption bands at 535 m/• flatten and eventually disappear (Fig. 1), and their iron content steadily decreases (Table I). The organic portion of the pig- ment has no characteristic absorption bands and is complexed in acid solution through its combination with iron. Therefore it is likely that when the iron content drops below a certain level (about 0.1%), the pig- ment becomes insoluble and cannot be extracted anymore. Indirect evidence for this view comes from Dutcher and Rothman's iron determinations in hair of different colors (13). Black and blonde hairs were found to contain, on the average, 2.71 and 2.43 mg Fe/100 g, respectively red hair, on the average, 9.78 mg/100 g i.e., about 7 mg more per 100 g hair than the other varieties. Assuming an iron content of about 0.5% in the original pigment in situ, 7 mg iron corresponds to 1400 mg pigment. Actually, red hair yields 40-60 mg trichosiderin per 100 g of hair therefore only 3-5% of the total amount may be ex- tracted. As the iron content of the pigment in the hair itself is unknown,
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)