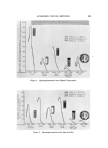
762 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS THEORETICAL CONSIDERATIONS Absorption of a photon of light results in raising of an electron from one of the low vibrational levels of the ground state, So, to one of the vi- brational levels of an excited singlet state, such as & or S2 (designated as process a on the Jablonski diagram, Fig. 1). Relaxation to a low vibra- tional level of the lowest excited singlet S• is usually very rapid (10 -•2 sec) and is shown as process b on the diagram. If the electron remains in S• for 10 -9sec or longer, the energy may be emitted as fluorescence (process c). Alternatively, the energy may be dissipated in a chemical reaction. Whereas in the singlet state the high energy electron retains its orig- inal spin, an inversion of this function may take place in a secondary step, referred to as intersystem crossing, to a triplet state T. This may take place directly to the lowest triplet, T•, shown on the diagram as process d, or by initial intersystem crossing to a higher triplet state, such as T2, followed by very rapid internal conversion to T• (radiationless process e). The lifetime of triplet states is usually much longer than that of the originally formed singlets and may be of the order of several seconds. Accordingly, many chemical reactions originate from triplet states. Other than via a chemical reaction, the triplet energy may be lost as heat or slow radiative emission, referred to as phosphorescence (process f). Direct absorption to a state such as T• or T• does not normally take place. However, T• can be generated directly by an interaction with another molecule which is in its triplet state, e.g., Tn, provided that ]'n ]'•. This phenomenon is known as sensitization. Singlet sensitiza- tion is also a common phenomenon in which the energy donor is in its singlet state. Conversely, loss of energy by one molecule to another is known as quenching (4, 5). • * T 1 RADIATIVE RADIAoeIONLESS Figure 1. Jablonski diagram
PHOTOCHEMISTRY IN COSMETICS 763 MEC}•A•XSMS OF P}•OTOSTAmI•Z•T•O• In the framework of these considerations, it can be seen that photo- stabilization is the process whereby one prevents photochemical teac- tions of molecules in their excited state. plished by interference with either: A. B. C. D. This objective can be accom- Absorption of light, Quenching of singlet S1, Quenching of triplet T•, or Prevention of chemical reactivity by changing the photochemical properties of the substrate. (This procedure is of limited interest as it alters the basic nature of the product.) In all cases, an additive is introduced which can take up the un- wanted energy and which can dissipate this energy as heat, as fluores- cence, or in a reversible chemical reaction. The above properties must be considered in conjunction with the obvious criteria involved in cos- metic formulation (toxicity, compatibility, cost, etc.). Most of the commercially available photostabilizers are of type A, i.e., ultraviolet light absorbers. The absorption of light by the additive is a function of its extinction coefficient at a given wavelength and that of the substrate to be protected. Thus, in the case of noninteracting solutions, eq 1, derived from Beer's law, can be used to calculate the percentage of light absorbed by the additive: % of light absorbed by the stabilizer = 100C• CASA + Cs•s CA and Cs are the molar concentrations of the additive and substrate, respectively. The •'s are the corresponding extinction coefficients at the wavelength in question. In concentrated solutions and in semisolid media the deviations from the above equation are parallel to the devia- tions from Beer's law. Nevertheless, the relationship is useful for esti- mates of the amount of additive to be used in a given preparation. The undesirable photochemical energy is dissipated in a reversible photochemical reaction. Thus, absorption of light may lead to a re- arranged structure which returns to the original form in a "dark," i.e., nonphotochemical, process. The following are examples of structural types and processes involved.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)