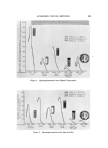
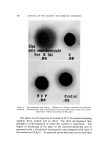
SUNSCREEN TESTING METHODS 823 of substance per area is not constant could account for the diverse re- sults. The water bath (//5) is a special test not designed to be correlated by rank in protection achieved rather, it shows that those substances which are readily water-soluble come off so easily that a S-minute gentle bath allows appreciable differences to accrue. For example, Figs. 1 and 2 (Method //5) show that water-insoluble Coppertone ø* sticks to the paper while water-soluble Sea & Skiøt is virtually washed off. The value of this method is as a test for screening substances which wash off in the usage situation. The other in vitro methods presented are valuable as industrial pro- cedures for estimating light absorbers' capabilities, but should not be used to serve as guidelines to dermatological products, since they do not simulate in vivo results. Only the in vivo ranking is detailed in this report (Table III). There is an inadequate patient population (three) to compare the protection achieved by one sunscreen with another. However, one can group these agents even using only three patients in an experiment proved statisti- cally valid. There is overlapping of groups because of the small patient population. ACKNOWI•EUGMENT The authors gratefully acknowledge the guidance in the use of statistics of Strother H. Walker, Ph.D., Biometrics Division, Depart- ment of Preventive Medicine, University of Colorado Medical Center. (Received March 7, 1969) REFERENCES (l) Daniels, F., Jr., Some comments and suggestions for the measurement of the protective effect of sunscreens, J. Soc. Cosmetic Chemists, 15,709-16 (1964). (2) Blum, H. E., Either, M., and Terus, W. S., Evaluation of protective measures against sunburn, Am. J. Physiol., 146,118-25 (1946). (3) Master, K. J., Sayre, R. M., and Everett, M. A., New evaluation techniques for sun- screens, J. Soco Cosmetic Chemists, 17,581-9• (1966). (4) Kodak Plates and Films for Science and Industry, 1st Ed., Eastman Kodak Co., Rochester, N.Y., 1967, p. 27d. (5) Form 590, Features and Specifications of the Burdick UV-800 Ultra-Violet Lamp, The Burdick Corp., Milson, Wis. (6) Geise, A. C., Cellular Physiology, W. B. Saunders & Co., Philadelphia, Pa., 1968, p. 201. * Coppertone Corp., Miami, Fla. t Sea & Ski Corp., Reno, Nev.
824 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (7) (8) (9) (10) (11) 12) 13) 14) 15) 16) 17) 18) 19) Moon, P., Proposed solar radiation curves for engineering use, J. Franklin Inst., 230, 583-617 (1940). Kahn, G., University of Colorado Medical Center, 1968, unpublished spectrophotometric data. Harber, L. C., Clinical evaluation of quantitative differences in UV absorption of com- pounds containing the substituted benzoic acid nucleus, J. Invest. Dermatol., 23,427-35 (1954). Daniels, F., Jr., and imbrie, J. D., Comparison between visual grading and reflectance measurements of erythema produced by sunlight, Ibid., 30,295-304 (1958). Van der Leun, J. C., Theory of ultraviolet erythema, Photochem. Photobiol., 4, 453-8 (1965). Blum, H. F., Radiation Biology, II, McGraw-Hill Inc., New York, N. ¾., 1955, p. 491. Rottier, P. B., Biologic problems concerning sunscreens, Y. Soc. Cosmetic Chemists, 19, 85-93 (1968). Keller, P., (•ber die Wirkung des ultravioletten Lichtes auf die Haut unter besonderer Berficksichtigung der Dosierung, Strahlentherapie, 16,824-35 (1924). Luckiesh, M., Application of Germical Erythemal and Intra Red Energy, Van Nostrand Co., New York, N.Y., 1946. Wilson, W. W., Quero, R., and Master, K. J., The search for a practical sunscreen, Southern Med. J., 59, 1425-30 (1966). Rossman, R. E., Knox, J. M., and Freeman, R. G., Acrylonitriles, a new group of ultra- violet absorbing compounds, J. Invest. Dermatol., 39,449-53 (1962). Giese, G. C., and Wells, J. M., Sunburn protection, natural and artificial, Sci. Monthly, 62,458-64 (1946). The Givaudian, Sindar Corp., New York, N.Y., 1961.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)