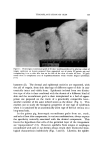
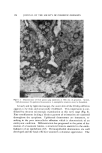
SUBCELLULAR COMPONENTS FROM HUMAN EPIDERMIS 97 nase was washed out of the epidermis with 0.25M sucrose. The tissue was homogenized in an all-glass homogenizer, and the homogenates were stained for microscopic examination. As determined by this procedure, the best loosening of the epidermal cells was obtained in a keratinase solution of 150 t•g/ml after 20- and 25-min incubation periods at 25øC. Preparation of Cell Mitochondria and Microsomes Sheets of epidermis were incubated for 20-, 25-, or 30-min periods with keratinase (150 t•g/ml). The epidermal sheets were then washed three times with 5 ml of 0.25M sucrose solution for 3 min in a mechani- cal shaker to remove the excess keratinase. All subsequent operations were carried out at 0-4øC. The epidermal sheets were cut into small pieces in 0.1M tris buffer, pH 8.0, in an Omnimixer* operating for 90 sec. This shredding facilitated the subsequent cell disruption with 10 passes in an all-glass Ten Broeck homogenizer* which was rotated be- tween 800-900 rpm. The homogenate was then transferred to a 15-ml centrifuge tube and centrifuged at 900 X g for 15 min to sediment the nondisrupted cells (all X g values are expressed for tip of centrifuge tube). The supernatant fluid was decanted very carefully so as not to disturb the residue. The residue was resuspended in buffer, again ho- mogenized in the Ten Broeck homogenizer, and centrifuged. The su- pernatant fractions were combined and then spun at 13,000 X g for 20 min in an International refrigerated centrifuge,* Model B-20, to sedi- ment the mitochondria. The mitochondria were washed two times by resuspension in 0.25M sucrose solution and then sedimented by centri- fuging as above. The final pellet of mitochondria was suspended in 0.25M sucrose. The supernatant fraction obtained after separation of the mito- chondria was centrifuged in a Spinco Ultracentrifuge,õ Model L, at 105,000 X g for 1 hour. The supernatant or cell-soluble fraction was decanted carefully. The microsomal pellet was resuspended in 0.25M sucrose, centrifuged again at 105,000 X g, and then suspended in the same solution. * Ivan Sorvall, Inc., Pearl St., Norwalk, Conn. 02054. ? Scientific Glass Apparatus Co., Inc., 735 Broad St., Bloomfield, N.J. 07003. • International Equipment Co., 300 Second Ave., Needham Heights, Mass. 02194. õ Beckman Spinco, 1117 California Ave., Palo Alto, Calif. 94304.
98 .JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Isolation o[ Nuclei Human epidermis was treated with keratinase as previously described. The tissue was then homogenized for 90 sec in an Omnimixer with 0.25M sucrose containing 1% citric acid. This was followed by ho- mogenization (with an all-glass homogenizer) in the same way as given above. The resultant homogenate was then layered over 0.44M sucrose which was layered over 0.88M sucrose, both containing 1% citric acid. This triple-layered material was centrifuged at 50 X g for 15 min in an International refrigerated centrifuge,* Model PR-1, which was acceler- ated and decelerated slowly in order to avoid mixing of the layers. The three layers were separated, centrifuged at 900 X g for 10 min, and the pellets were examined microscopically. The top layer contained some nuclei and small pieces of keratin, the middle layer contained mostly free nuclei and a few cells, and the bottom layer had large pieces of keratin and some clumps of cells. Nuclei obtained from the top and middle zones of the above fractionation were resuspended in 0.25M sucrose in 1% citric acid, and again layered over 0.44M sucrose which was layered over 0.88M sucrose, both containing 1% citric acid. This mixture was centrifuged at 50 X g for 25 min and then examined microscopically. The middle layer contained essentially pure nuclei. This nuclear-rich fraction was washed three times with 0.25M sucrose containing 0.0009M CaC1.., (9), and finally suspended in about twice its volume of the sucrose- CaCI_• solution. The total nitrogen content of the epidermal homogenates and the various cell fractions was determined by a micro-Kjeldahl method. Enzyme Assay o/ the Epidermal Fractions The marker enzymes were: cytochrome oxidase for mitochondria reduced nicotinamide adenine dinucleotide phosphate (NADPH)-cyto- chrome c reductase and glucose-6-phosphate dehydrogenase for micro- somes and nicotinamide adenine dinucleotide (NAD)-pyrophosphory- lase for the nuclei, in both keratinase-treated and untreated samples of epidermis. The activity of these marker enzymes was used to determine whether keratinase induced sufficient loosening of the epidermal cells so that homogenization increased the relative amount of particulates, and to ascertain whether keratinase had any deleterious effect on the ac- tivity of these enzymes. The activities of the various marker enzymes * International Equipment Co., 300 Second Ave., Needham Heights, Mass. 02194.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)