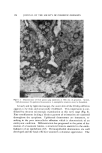
SUBCELLULAR COMPONENTS FROM HUMAN EPIDERMIS 105 extraneous coat from sarcoma 37 cells. In this report, the incubation of human epidermis with keratinase at 25øC has been shown to help in the separation of epidermal cells, apparently by dissociating the epidermal intercellular keratinase-like material. Clarification of some aspects of enzyme histology and enzyme chem- istry of human epidermis has been made possible by various investiga- tors (19-21). Several authors have established the presence in the human skin of the three major pathways for the oxidation of glucose these are the Kreb's tricarboxylic acid cycle, the glycolytic pathway, and the hexose monophosphate shunt. Of the oxidative enzymes in the epidermis, cy- tochrome oxidase has been studied extensively. The cytochrome oxi- dase activity of mitochondria isolated from keratinase-treated and un- treated human epidermis showed no significant difference (Table II). However, the mitochondrial preparations contained appreciable nonmi- tochondrial, fragmented, particulate matter such as keratohyalin, des- mosomes, etc. (Fig. 6). The separation of the mitochondria from these particulates poses some difficulties unless all the components remain in- tact during homogenization. Of particular importance is the procure- ment of sufficient fresh skin (or any other tissue) for the isolation of the cell particulates (14). The activity of cyt.ochrome oxidase in human epi- dermis is much lower than that found in rat liver (22), and lower than that found in mouse epidermis (23). Recent experiments have also demonstrated the presence of two other enzymes in human epidermis namely, purine nucleoside phos- phorylase and cytochrome c reductase (19). NADPH-cytochrome c re- ductase and glucose-6-phosphate dehydrogenase are exclusively associ- ated with microsomal membranes (24), hence they were used in this re- port as a marker for the microsomal preparations. The specific activity of NADPH-cytochrome c reductase was rather low but fairly constant in keratinase-treated and untreated human epidermal microsomes (Table II) even though these structures were fragmented and ribosome-free (Fig. 4). Only a trace, if any, activity was reported in six samples of normal mouse epidermis (2g), whereas the microsomes of normal rat liver show appreciable NADPH-cytochrome c reductase activity (24). The activity of glucose-6-phosphate dehydrogenase was shown to be associated with microsomal membranes in rat liver (24). In human breast epidermis, however, the activity was found in the soluble super- natant fraction (Table II), possibly due to solubilization from the mem- branes. This may result from the unavoidable delay in securing fresh human skin and, as a consequence, the disintegration of the membranes
106 .JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS and the subsequent liberation of this enzyme into the supernatant frac- tion (14). The possibility exists, however, that glucose-6-phosphate de- hydrogenase is not attached to the microsomal membranes of epidermis. In general, the preparation of nuclei from human epidermis was sat- isfactory in that very few whole cells were present (Fig. 3). However, a small amount of particulate matter, probably keratin, could be observed in most nuclear preparations, even after repeated washing by gradient centrifugation. This procedure removed most of the soluble constituents of the cytoplasm. As mentioned above, methods for assessing cytoplasmic contamination of enzymes of one subcellular component by those of another are not generally helpful in a tissue such as epidermis which has low activities of many enzymes. However, cytochrome oxidase activity was determined in the nuclear fraction of epidermis and found to be nil, showing the absence or trace only of mitochondria. The experi- ments with isolated nuclei are rendered more difficult by the paucity of nuclear marker enzymes which have been studied in detail. Two en- zyme activities that are largely concentrated in the nuclear fraction are NAD-pyrophosphorylase and DNA-dependent RNA-polymerase. Re- cent research with mitochondria has shown that even the latter enzyme is not unique to the nucleus. In this report, the results indicate that NAD-pyrophosphorylase activity of keratinase-treated and untreated nuclei showed no consistent differences (Table III). ACKNOWLEDGMENTS The authors are grateful to the Department of Pathology at Buffalo General Hospital and to the Departments of Breast Surgery and Pathol- ogy of Roswell Park Memorial Institute for the human skin samples. They are also indebted to Drs. U. Kim and J. Horoszewicz of this Insti- tute for the electron microscopy. (Received August 10, 1970) REFERENCES (1) Sanadi, D. R., and Fluharty, A. L., On the mechanism of oxidativc phosphorylation. VII. Thc energy-requiring reduction of pyridine nucleotide by succinate and the en- crgy-yielding oxidation of reduced pyridine nucleotide by fuma•'ate, Biochemistry, 2• 523-8 (1963). (2) Chance, B., and Hagihara, B., Direct spectroscopic measuremcnts of interaction of com- ponents of the respiratory chain with ATP, ADP, phosphate, and uncoupling agents, Proc. Int. Congr. Biocbem., 5th, Moscow, 5, 3-37 (1961). (3) Duell, E. A., Inoue, S., and Utter, M. F., Isolation and properties of intact mitochondria from spheroplasts of yeast, J. Bacteriol., 88, 1762-73 (1964).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)