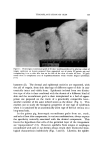
PHOTOCHEMICAL REACTIONS 91 Photodimerization For naphthoquinones such as menadione, ultraviolet radiation in- duces photodecomposition, as indicated by decrease in UV absorbance, which apparently involves formation of dimers and higher polymers as an important decomposition pathway. Miceliar surface active agents such as sodium decyl sulfate increase the rate at which this process proceeds, as indicated in Fig. 6. With reagents specific for the quirtone function, as well as measurement of UV absorbance, it appears that the surfactant increases the rate of the dimerization process, rather than the rate of other photochemical reactions involving hydrogen abstraction by the quinone. Menadione binds to sodium decyl sulfate in the dark (ground state) as indicated by the solubility data presented in Fig. 7. The marked in- crease in rate of dimerization relative to rate of hydrogen abstraction in presence of sodium decyl sulfate suggests that the enhanced rate of dimer formation may be a result of increased approximation of excited state and ground state menadione species in the miceIlar phase. 1.0 0.9 O.B 0.7 0.6 ANAEROBIC I.O 0.9 0.8 0,7 0.6 I 0 20 30 4 0 SECONDS AEROBIC 0 I I I I I0 20 30 40 SECONDS Figure 6. Influence of sodium. decyl sulfate on late of photodecomposition ooe 4.5 to 5.8 X 10-*M menadione in distilled water, 25øC. A, no additive B, 2% sodium decyl sulfate
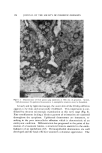
92 .JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS TOTAL 5 FREE 4 I I I I i 2_ 3 4 SODIUM DECYL SULFATE (%W/V) Figure 7. Binding of menadione to sodium decyl sulfate, as determined by solubility of menadione in presence of SDS, 25øC. Discontinuity in plot corresponds to critical micelie concentration for SDS Energy Transfer Reactions Evidence of triplet-triplet energy transfer reactions in aqueous solu- tion was first presented by Posthuma and Betends in 1961 (22), involving absorption of visible light by riboflavin and destruction of the polyene fungicide Pimaricin. These authors also studied the effect of dyes such as fluorescein, proflavin, lumichrome, acridine orange, eosin, and erythro- sin on the isomerization of substrates such as stilbene 4-carboxylic acid and cinnamic acid (23). Sensitizers possessing a triplet level higher than or equal to that of the acceptor may transfer their energy to the acceptor. A typical energy transfer reaction is illustrated by the riboflavin photooxidation of the acridan drug, Clomacran (I) [2-chloro-9-(3-di- methylaminopropyl)acridan phosphate], to its acridine derivative (II) •CH• (C. H•)•N cH• C1 (CH2)aN. C1 ••C (I) , (II) I H (24).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)