INTERMEDIATES FOR HAIR COLORS 141 Perhaps the most important difference in the behavior of these nitro dyes before and after recrystallization is their effect on ageing when dis- solved or dispersed in the dye base solutions. The composition of the base solution varies with each manufacturer of oxidation hair dyes. A detailed discussion of oxidation hair dye base solutions has been given in a prior publication (2). In the experimental work done for this paper the following base solution (amounts given in grams) was used: Dye 0.1 Oleic acid 20.0 Ethomeen S-12' 15.0 Propylene glycol 11.0 Isopropyl alcohol 12.5 Ammonium hydroxide (28 %) 10.0 Sodium sulfite 1.0 Hampshire DEG•' 1.5 Water (deionized) 29.0 The pH of the base solution is 10.4 and after the addition of an equal volume of hydrogen peroxide it is 9.7 to 9.9. The dye is dissolved in propylene glycol by heating. The Ethomeen and oleic acid are mixed together and the dye solution is added with stirring. Next, the isopropyl alcohol is added, then the ammonium hy- droxide. The sodium sulfite and Hampshire DEG are dissolved in the water and this solution is added slowly, with stirring, to the above solu- tion. Dispersions or solutions of the intermediates, before and after re- crystallization, were prepared at the same time. Results are discussed separately for each of the five compounds considered in this paper. In another test, the behavior of the crude and purified dyes on boil- ing in water was investigated by the following procedure: I g of dye was added to 400 ml of boiling deionized water and the solution was boiled on a hot plate for exactly 5 minutes. It was filtered through a weighed fritted glass filter with a base of medium porosity. The precipitate was washed by filling the crucible with boiling water, allowing it to drain completely between fillings, and continuing the washing until the tiltrate was no longer colored. The percentage residue was quantitatively de- termined after drying. Laboratory recrystallizations were performed with all five products and the substances removed during the hot filtra- tion were used for further examination. * Ethomeen S-12 is bis-2-hydroxethyl soybean amine, Armour Industrial Chemical Co., Chicago, Ill. * Hampshire DEG is diethanolglycine sodium salt, Hampshire Chemical Co., Nashua, N.H.
142 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS RESULTS Nitro-o-phenylenediamine Nitro-o-phenylenediamine can be prepared by the reduction of 2,4- dinitroaniline with alcoholic ammonium sulfide (3). The solubility at 25øC in aqueous solution at a pH of 9.7 is 0.03% (1). Comparable analy- ses of this intermediate before and after recrystallization, as a production lot, are given in Table I. Table I Analyses of Nitro-o-phenylenediamine Purified Raw PuriW, % 99.5 95.4 Ash, % 0. 025 0.20 Iron, ppm 36 178 Melting point (Fisher-Johns), øC 202.5 199.4 Base solutions were prepared using the formulation previously shown under Experimental. One of these contained 0.1% of the crude nitro-o- phenylenediamine and the other, 0.1% of the purified product. Both solutions were clear and showed no precipitation at the time of bottling. They were aged in sealed bottles, at room temperature and at 50øC, and examined from time to time for precipitation. Precipitation in the solu- tion containing the crude product started after 24 hours, both at room temperature and at 50øC, while no precipitate appeared in bottles con- taining the purified material after ageing for four weeks. Infrared spec- trograms for crude and purified nitro-o-phenylenediamine did not show any differences. The material removed in the laboratory recrystallization of nitro-o- phenylenediamine during hot filtration of the isopropyl alcohol solution was washed repeatedly with hot isopropyl alcohol until the flitrate came through colorless. From the recrystallization of 100 g of crude nitro-o- phenylenediamine 0.5 g of sludge was obtained. After drying, it was a black powder with a melting point above 335øC, with an ash content of 16.3% and an iron content, determined as ferric oxide, of 3.77%. An infrared spectrogram of this black powder is shown in Fig. 1.* * All spectrograms presented in this paper were prepared on a Perkin-Ehner Model 137B Inoeracord spectrophotometer using the potassium bromide pellet method with slit N and a fast scan. A concentration of 0.5% intermediate and a pellet thickness of 0.022 in. was used.
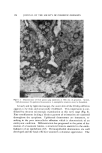
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)