FETAL EPIDERMIS 12l equilibrated over Drierite, ©* and the steady-state diffusion rates were measured at 25 øC. Determination o[ Epidermal Sul[hydryl Histochemical Portions of fetal skin were fixed in ethanolic trichloroacetic acid for histochemical demonstration of protein-bound sulfhydryl groups accord- ing to the DDD method of Barrnett and Seligman (6). In this method, sparse, widely-separated sulfhydryl groups react with the reagent to prod- uce a red or pink color (monocoupling) a blue color (dicoupling) indi- cates a •-eater concentration of sulfhydryl groups. Biochemical Each fetus was rinsed in physiological saline and in water, then skinned, and the epidermis was separated following exposure of the skin to ammonia vapor (7). Animals in an advanced stage of development were visibly hairy, a condition which necessitated epilation prior to am- monia treatment. Molten Paraplast ©* (56øC) was poured over the hairy skin surface and allowed to harden. The skin was then forcibly sepa- rated from the paraffin in which the hairs were trapped. Epidermal sulfhydryl (--SH) was determined using a modification of the Flesch and Kun method (8). The modified method involves measur- ing the disappearance of the --SH reagent, 1-(4-chloromercuriphenylazo)- naphthol-2 (CMPAN). The CMPAN was synthesized by the Lever Or- ganic Section using the procedure of Bennett and Yphantis (9). Deter- minations were made of both "free" --SH by reaction in water and of "total"--SH ("free" plus "masked") by reaction in 3M urea (10). The level of total --SH was found to be independent of urea concentration within the range 3-8M. To prevent oxidation of --SH by cyanate (11), the urea solution was freshly prepared. The modified method requires as little as 1 mg of epidermal tissue and is most suitable for use in the range 10 s to 10 -7 moles of sulfhydryl sulfur. A description of the modi- fied procedure follows. The reaction was carried out near 0øC in a 1.5-ml volume of either water (free --SH) or 3M urea (total --SH). All samples were first homog- * Drierite,© W. A. Hammond Drierite Co., Xenia, Ohio. t Paraplast© Tissue Embedding Medium, Sherwood Medical Industries, Inc., 1831 Olive St., St. Louis, Mo. 63103.
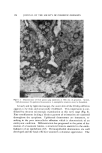
122 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS enized in water. To the samples designated for analysis of total --SH, 12M urea was added in such quantity as to bring the final urea concen- tration to 3M. The concentration of tissue in the reaction vessel ranged from 2 to 25 mg/ml. Four milliliters of the --SH reagent (1.5 mg % CMPAN in amyl ace- tate) was added to each tube and the tubes were agitated for 20 minutes in a shaker. The tubes were centrifuged and the absorbancy of the organic (upper) layer was determined at 500 nm. The --SH content of the sample was calculated from the relation AXVXSX]O -a a,,• X W = •g sulfhydryl sulfur/mg tissue where A -- decrease in absorbancy of CMPAN solution V -- volume of CMPAN solution in ml S -- at. wt sulfur (32.07) W -- dry weight (105øC) of tissue sample in mg a,, -- molar absorbancy index of CMPAN solution at 500 nm -- 1.58 X 104 in the range 0-33/•g/ml (l-cm cells) Electron Microscopy Pieces of fetal skin were fixed in 1% phosphate-buffered osmic acid, washed thoroughly with distilled water, and dehydrated with a graded series of alcohols. After immersion in propylene oxide, the tissue was embedded in Epon ©* 812 contained in gelatin capsules and the Epon was allowed to polymerize at 60øC. Sections were cut on a Porter-Blum ul- tramicrotome* with a diamond knife, mounted on carbon-reinforced collodion-coated grids, and stained with 1% aqueous phosphotungstic acid. Microgzaphs were taken with a Philips EM-100B electron micro- scope,* using Kodak spectographic fihn. Light Microscopy Tissue for light •nicroscopic exa•nination was fixed in buffered forma- lin and e•nbedded in Paraplast. Sections were cut at 4 • and stained with he•natoxylin and eosin, Van Gieson's stain, Massoh's trichrome stain, and reacted for carbohydrates using the periodic acid-Schiff procedure. * Epon© Resin 812, Shell Chemical Co., New York, N.Y. ? Porter-Blum Ultramicrotome MT2, Ivan Sorvall, Inc., Norwalk, Conn. $ Philips Electronic Instruments, 7.50 So. Fulton Ave., Mt. Vernon, N.Y.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)