]. Soc. Cosmet. Chem., 25, 99-114 (February 3, 1972) Clear Zinc Pyrithione Preparations TERRY GERSTEIN, M.S.* Presented May 24, 1971, Seminar, Washington, D.C. Synopsis--ZINC PYRITHIONE, also kn,own as zinc pyridine-2-thiol-l-oxide, has been es- tablished as an effective ANTISEBORRHEIC AGENT. Because of its limited aqueous solubility it is currently being marketed as a suspension in hair-cleansing and hair-grooming preparations. A method has been developed in which clear, aqueous (as well as nonaqueous) products can be prepared. The method is based upon the COMPLEXATION of zinc pyrithione with certain ORGANIC AMINES. It appears that zinc pyrithione is highly soluble in many primary aliphatic amines. In other more complex amines, such as the poly- alkylenimines, a definite stable molecular complex is formed. The ability of such amines to complex and produce a soluble form of zinc pyrithione is discussed with respect to the physical and d•emical properties of the ensuing solutions. Based on these findings, CLEAR HAIR Pi•EPARATIONS containing zinc pyrithione can be prepared for use as antidandruff cleansers, rinses, grooming agents, and conditioners. Ex- amples are offered of product formulations having levels of zinc pyrithione ranging from 700 to 20,000 ppm (2%). Analytical data concerning complex formation as well as the complexes' toxicology are presented. INTRODUCTION Zinc pyrithione, also known as zinc pyridine-2-thiol-l-oxide (ZPTO), has been established as an effective antiseborrheic agent (1). It is currently marketed in shampoo and hair dressings at concentration levels of about 0.5% in ,the hair dressing to about 1•o in the shampoo. This active ingredient is present in the form of a dispersed solid in these products since its solubility is quite limited in both water and oil media. The literature states that its solubility in water is 10-20 ppm at pH 7 and increases to 35-50 ppm at pH 8 (2). In ethanol, the solu- bility is 290 ppm in acetone, 700 ppm in benzene, 3-5 ppm and in diethylether, 0 ppm. In other solvents, the solubility increases--for * Revlon Research Center, Inc., 945 Zerega Ave., Bronx, N.Y. 10473. 99
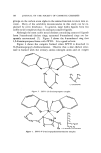
100 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS example, in chloroform, ,to 0.34% and in dimethylformamide, to 0.81%. The solubility in dimethylsulfoxide is reported to be 5.13% (2). Ac- cordingly, it has been difficult to formulate suitable cosmetic composi- tions containing solubilized ZPTO. Although the sodium salt of pyrithione is highly soluble in water, and like ZPTO has been found to have fungicidal and bactericidal properties, it, unlike ZPTO, has been found toxic and unacceptable for use in cosmetics or dermatological compositions for topical application to the skin (3). HYPOTHESES It was postulated that the solubility of ZPTO would be increased in solvents containing amino functional groups. The reasoning for this is based upon the ability of certain heavy metals such as cadmium, zinc, copper, and nickel to form ammine complexes. Zinc has a coordination number of 4 which means that it can attach itself spatially to 4 ligand linkages besides maintaining its two primary valence linkages. In the molecular structure of ZPTO (Fig. 1), two of the four available spatial positions are filled by the oxygen in the oxide functional groups of the molecule. A chelate is formed. By selecting the proper amine solvent it was hoped that the chelate structure would be modified to allow for the accommodation of a molecular complex containing amino groups that would utilize the remaining two Werner bonds. Figure 1. Zinc pyrithione (zinc pyridine-2-thiol-l-oxide) (ZPTO) One of the first materials to be tested was reagent grade 28% am- monium hydroxide. It was found that ZPTO was soluble in it to the order of 1 or 270. Although this in itself was not considered significant, it proved somewhat interesting when a 1% solution of ZPTO in am- monium hydroxide was used to neutralize aqueous Carbopol 941.* A clear gel resulted that had a pH of 8 and contained 100 ppm or 0.01% ZPTO. When the pH of the gel system was lowered, it was found that * B. IV. Goodrich & Co., Cleveland, Ohio.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)