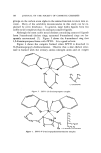
ZINC PYRITHIONE PREPARATIONS 109 Figure 8. Possible structures of solubilizc{ ZPTO in aqueous PEI planarions. The first (Fig. 8,A) is that a complex is formed with PEI that is analogous to the tetraammonia zinc complexes. For this to happen the two coordination linkages that form the internal chelate structure of ZPTO would have to open and allow the total of four co- ordination linkages of zinc to form with PEI. The second explanation (Fig. 8,B) is that of a decomposition of ZPTO into a pyrithione salt of PEI and zinc which would be complexed in alkaline PEI media. It• this is the case, then a solution of ZPTO in aqueous PEI may exhibit toxic pharmacological properties similar to sodium pyrithione and therefore render its use in cosmetic compositions unacceptable. To determine the nature of the PEI-solubilized ZPTO, a series of chromatographic techniques was employed in which gel filtration studies were prominent. Gel filtration, a form of column chromatography, separates substances by molecular weight. The higher the molecular weight, the more rapidly the substance is eluted from the column. ZPTO has a molecular weight of 318 compared to approximately 600 for the PEI which we used. If the two are not complexed they should separate on the column into two peaks. It was found that PEI, zinc, and pyrithione migrate as one peak. This phenomenon indicates that the zinc and the pyrithione exist in chemical combination in the PEI sys- tem. This conclusion was also sustained from data obtained from thin- layer chromatography studies.
110 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Extensive toxicological studies confirm the existence of an intact ZPTO molecule. A subacute percutaneous toxicity study on the intact skin of white albino rabbits was performed with a 2% solubilized ZPTO solution, pH 8.8. This 90-day study showed that ,there were no demon- strable systemic effects produced by the repeated application of the prod- uct on skin at greatly exaggerated levels of intended human use (11). Corresponding tests with equivalent levels of sodium pyrithione did pro- duce systemic toxicity. One of the first formulations prepared containing the ZPTO-PEI complex was a clear liquid hair dressing with a ZPTO concentration of 0.5% (Table II). This formulation was based upon an alkoxylated liquid having hydrophylic-hydrophobic groupings. Included in the formulation were natural and synthetic esters, ethoxylated glycerine, and other ingredients that we thought would contribute to a balanced hair dressing. The total nonvolatile solids content was 20%. It was decided to determine clinically if the ZPTO-PEI complex in this product base was an effective treatment for seborrheic dermatitis. Before doing this, the preparation was submitted to a series of pharmacological and toxicological tests to which new products are subjected in order to maintain maximum consumer safety. After the safety of the prepara- tion had been established it was then offered for clinical testing in Europe. Tests were conducted concurrently by dermatologists in Great Britain, France, and Italy. The results of all these tests indicated that the preparation was definitely effective in alleviating dandruff and, in many instances, in controlling even the most severe cases (12). Table II Clear Liquid Hair Dressing Sequence Ingredient Per Cent 95% ethanol 71. 697 Ucon fluid mixture a 13.5 Castor oil 2.0 Diisopropyl adipate 0.5 Liponic EG-I• 2.0 UV light absorber 0.05 Perfume oil 0. 313 Water 7.0 'ZPTO 0.5 PEI 1.0 (Water 1.0 3.7% hydrochloric acid 0.44 (q.s.--pH 8.9) Union Carbide Chemical Co., New York, N.Y. Lipo Chemicals Inc., New York, N.Y.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)