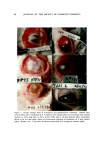
ZINC PYRITHIONE PREPARATIONS 107 ".•.....•. - ß e.!: ... : •dl..:: :.• .. • .• .• ., •:: ' '."' 2-'- • .•:• :_:.:•::• .': ......... . ........... ....,.j: ... . . .. .:•:-..:-. :•,:. ...... •.-••,..•:•- .... .. .:Z :•. "' , . '• • ..... ....•..: . • :• :,... •. }. ß .:• ,i.' '.• . • ...... :' ' "'•' ' .. • ._. •- .• .& .--.,-,•?.....::.... :::- . ::. . --:.:. -....:.: ..:-: :---.?..:':-: :.. • •. .'1 .... .. . ..... . •.•'.• :. '.,¾i ,..:.:. . :..•, , % ........ : - • -.•: ....... •,.... Figure 6. The an:monium hydroxide-Carbopol gel (left) contains 200 ppm ZPTO and is cloudy. The diglycolaminc-Carbopol gel (right) contains 700 ppm ZPTO and appears clear, due to an optical effect of the crystals and media in the ammonium hydroxide-Carbopol prepara- tion, thus making these crystals more difficult to observe. These crystals will grow in size but the preparation will continue to appear clear. By altering the refractive index of the media the crystals will be more visually pronounced and may give an unusual sparkling effect to the product. Neither the efficacy nor the toxicological properties of the for•nulations outlined in Table I have been determined. Polyethylenimine As the search for other amino solvents progressed, we became in- volved with a group of amines that were decidedly different in their properties: the polyalkylenimines and particularly the polyethylen- imines. This group was especially attractive in that their higher mo- lecular weight and bulky molecular structure made them relatively in- vulnerable to subcutaneous absorption, a particular liability of many smaller organic amines (8). Polyethylenimine is made by the acid-cata- lyzed polymerization of aziridine (Fig. 7). The resulting cationic poly- mer, depending upon polymerization conditions, can have a molecular weight as high as 100,000 and have as a composition a constant ratio of 25% primary amine to 50% secondary amine to 25% tertiary amine (9).
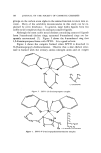
108 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS AZIRID•NE H POLYETHYLENIMINE (PEI) oeigure 7. Acid-catalyzed polymerization ooe aziridine to form polyethyleneimine What made this material unusual as compared to other amines which had been tested was the fact that water or alcohol was actually required in order to dissolve ZPTO. In effect, we were now able to make clear preparations of ZPTO that contained large quantities of water. Ini- tially, it was found that 1 part ZPTO can be conveniently dissolved in water using 2 parts polyethylenimine of molecular weight 600 (PEI). The resulting solution, based upon a 20% ZPTO concentration, had a pH of 10.6 and could be diluted to infinity with water without causing the precipitation of ZPTO. It was later determined that 1 part ZPTO can be so]ubilized with as little as 1.35 parts PEI. When the pH of an aqueous solution containing 270 ZPTO and 470 PEI was lowered by the slow addition of 3.7•o hydrochloric acid, a precipitate occurred be- low a pH of 8.8. The precipitate was identified as ZPTO by infrared spectroscopy and was shown to have the same spectra as the crystals growing in the diglycolamine-Carbopol hair dressing. In a study of the structure of the complex it was shown that the ultraviolet absorption spectrum of a dilute aqueous solution of ZPTO shifts to one similar to sodium pyrithione when aqueous PEI is added (10). Since sodium pyrithione is an ionic salt and has no coordinate covalent metal linkages, the indication is ,that the chelate bonds within the ZPTO molecule are broken. From this inference, the solubilization of ZPTO by aqueous PEI can be accounted for by either of two ex-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)