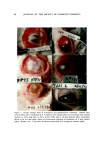
ZINC PYRITHIONE PREPARATIONS Table III Product Formulations Based upon the ZPTO-PEI Complex 111 Sequence 1 1 1 Sequence 1 1 Sequence 1 1 Clear Shampoo Ingredient Sandopan DTC Acid• Lauric alkanolamide Water ZPTO PEI Water Clear Protein Hair Conditioner Ingredient Water-soluble protein Water ZPTO PEI Water Perfume-hydrotrope 3.7% hydrochloric acid Hair-Thickening Lotion Ingredient Polyvinylpyrrolidone K-30 Water 'ZPTO PEI Water 'Methocel resin b •Water 3.7% hydrochloric acid Per Cent 20.0 3.0 67.0 2.0 4.0 4.0 Per Cent 1.0 97.9 0.2 0.4 0.4 0.1 q.s.--pH 8.8 Per Cent 7.0 40.5 0.5 1.0 1.0 1.0 49.0 q.s.--pH 8.8 Sandoz Chemical Industries, Hanover, N.J. Dow Chemical Co., Midland, Mich. A series of prototype formulations of other hair products was pre- pared (Table III). In many cases the concentration levels of ZPTO are unrealistically high in order to demonstrate the ease of incorporating a large quantity of the complex. The clear shampoo formulation shown in Table III contains Sandopan DTC Acid which is an ethoxylated fatty alcohol material having, in addition, a carboxylic acid function. This acid surfactant is used to neutralize some of the excess PEI that is available when the ZPTO-PEI complex is formed. The pH of the shampoo may be as low as 8.8 without affecting clarity. This shampoo exhibits good foaming properties. In place of the Sandopan DTC Acid other acidic surfactants may be used, such as the phosphoric acid esters of ethoxylated fatty alcohols or
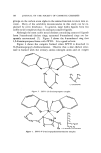
112 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS the succinic acid half-esters of fatty amides. The use of ingredients containing sodium ion should be avoided because of the possibility of hydrolizing the ZPTO at high pH's and forming the sodium pyrithione in situ. Other prototype hair preparations made (Table III) were a collagen- derived protein conditioner containing 0.2% solubilized ZPTO and a clear hair-thickening lotion based upon polyvinylpyrollidone that con- tained 0.5% solubilized ZPTO. Both of these preparations had a pH of 8.8. Related Amines Tested Besides the polyethylenimine, molecular weight 600, used in the previous formulations, it was found that polyethylenimines of higher molecular weights are also suitable. Polyethylenimines, for example, of molecular weights of 1,200, 1,800, and 60,000 were able to solubilize ZPTO in water. When the polyethylenimine function is ethoxylated or propoxylated, thus reducing the primary amine content, the solubilizing effect on ZPTO is reduced. If a modification of the polyethylenimine molecular structure is made so as to yield a linear polymeric molecule composed of only secondary amine functions, it has no solubilizing effect on ZPTO. It was found that when the molecular weight of polyethylenimine is reduced to g00, ZPTO is readily dissolved without the presence of water. Water can still be added to this solution without causing the precipitation of ZPTO. This was true of amines of even lower molecu- lar weight that may be considered to be derived from sections of the polyethylenimine molecule. Thus, tris(2-aminoethyl)amine and tetra- ethylene pentamine (Fig. 9) showed similar behavior to polyethylen- TRIS(2-AMINOETHYL)AMINE TETRAETHYLENE PENTAMINE Figure 9. Formulas for tris(2-aminoethyl)amine and tetraethylene pentamine
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)