GAS CHROMATOGRAPHY OF METHYLENE CHLORIDE 117 of agc and the volatilized gas was chromatographed. The discussion following the presentation of the paper suggested that the method could be applied to the determination of methylene chloride. Several workers reported using can-piercing devices and liquid sam- pling valves to inject samples directly into the gas chromatograph (8-10). This eliminated determination of temperatures and pressures, vapor pressures of various components in aerosol systems when gaseous sam- ples were taken, and eliminated the use of elaborate and expensive vacuum sampling devices such as previously discussed. However, liquid sampling valves are expensive, and the aerosol can must be retained with the puncturing device in place, or discarded. Cohen (11) eliminated the liquid sampling valve by using a Pre- cision Sampling Corporation Pressure-Flo©* high-pressure liquid sam- pling syringe. He modified the can-piercing device to sample the liquid in the can directly through a fitted filling adaptor. This reduces the cost of sampling to below $100 but, again, it does destroy the sample container by piercing. Bourne and Murphy (10) separated ethanol and methylene chloride from a number of propellants, both fiuorocarbons and hydrocarbons, on a series Carbowax 20M* and porous polymer column. However, they never attempted the separation of methylene chloride, ethanol, and Pro- pellant 11 in the same system. Cannizaro and Lewis (9) separated these three components on long (9.2 m) columns packed with 20% Halloomid M-18.* Under the conditions given in their procedure, methylene chlo- ride eluted in 13.5 min. By increasing the temperature of the column oven the retention time of the methylene chloride was shortened. In our work, we chose to use the porous polymer columns because the work of Bourne and Murphy (10) and our own experiments had shown we could achieve quick, clean separations of the propellants and solvents found in hair sprays. Because Par II©,õ the column previously used (10), is no longer available, we used Porapak©ll instead. Our re- sults showed incomplete separation of the Propellant 11 and methylene chloride. Increasing the length of either the Carbowax 20M or the porous polymer column or reducing the mesh size of the porous polymer * Precision Sampling Corporation, P.O. Box 15119, Baton Rouge, La. 70815. • Polyethylene glycol, tool wt ca. 20,000, Union Carbide Chemicals Co., 270 Park Ave., N.Y. 10017. $ Applied Science Laboratories, Inc., P.O. Box 440, State College, Pa. 16801. õ Hewlett-Packard Co., Route 41, Avondale, Pa. 19311. II Waters Associates Inc., 61 Fountain Street, Framingham, Mass. 01701.
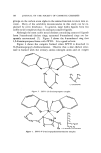
118 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Figure 1. Exploded view of nondestructive transfer needle assembly did little to improve the Propellant 1 l-methylene chloride resolution, but it did increase the retention time drastically. However, by placing a Carbowax 20M section before the Porapak Q section and following it with another Carbowax 20M section, the methylene chloride was com- pletely separated from the Propellant 11. A nondestructive sampling system was quite simply prepared from a Precision transfer button, a 22-gauge Luer hypodermic needle, and a piece of aerosol valve dip tube. A 1/2-in. and a 8/82-in. piece of the dip tube was cut off with a razor blade. The longer piece was put into one side of the transfer button and the needle fitted on it. The smaller piece was put in the other side of the button if the smaller diameter (882 in.) valve stem is to be fitted. This is illustrated in Fig. 1. The sample was taken in 10-ml serum bottles fitted with a stopper. A Precision Sampling Corporation Pressure-Flo high-pressure liquid syringe was used to transfer the sample into the injection port of the gas chromatograph. EXPERIMENTAL The gas chromatograph used for this work was a Hewlett-Packard 5750B series instrument equipped with a thermal conductivity detector. The detector current used was 150 ma temperature of the detector was 255øC and of the injection ports, 2g0øC. Helium at a flow rate of .gO cc/min was used as the carrier gas. The column used consisted of g portions: 1. 0.935 m X 3.2 mm (3 ft X 1/8 in.) 15% Carbowax 20M -{- 4% KOH on 60-80 mesh Gas Pack F,* 2. 2.45 m X 3.2 mm (8 ft X 1/8 in.) Porapak Q, 50-80 mesh, 3. 1.23 m X 3.2 mm (4 ft X 1/s in.) 15% Carbowax 20M -{- 4% KOH on 60-80 mesh Gas Pack F. * Chemical Research Services, Inc., 14 Industrial Road, Addison, Ill. 60101.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)