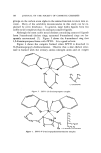
GAS CHROMATOGRAPHY OF METHYLENE CHLORIDE 123 A R E 2.0 A 1.0 Me CL z/n, - PROPANOL I.O 2D 5.0 WEIGHT Figure 4. Response of detecto,' to methylene chloride relative to n-propanol over range ooe ale, proximately 3 to 20% in hair spray It was observed that samples in the serum bottles could be inverted in a small beaker and held several hours at room temperature without change in composition. Maintaining these samples overnight, either at room temperature or at refrigerator temperature, was not successful. •towever, a series of samples held overnight in a freezer gave identical results when reanalyzed the following day. After 30 or 40 samples were run through the column, a slight distor- tion of the n-propanol peak and deteriorating resolution were observed. This was atributed to a build-up of plasticizer and other higher boiling ingredients in hair spray on the column. Conditioning the column at 200øC for 1/2 hour at the start of a day's run was found to eliminate this problem. There has been no indication as yet of any failure of the column after almost 500 analyses. SUMMARY A new gas chromatographic method for determining methylene chloride in hair sprays in less than 10 min has been developed. The reproducibility of commercial samples containing 7-8% methylene chlo- ride has been shown to be better than --2% relative. Expensive sample equipment or instrument modifications are not required and training of personnel is minimal. (Received October 1, 1971)
124 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS P•EFERENCES (1) Aerothene Chlorinated Solvents Product Bulletin, The Dow Chemical Co., Midland, Mich., 1967, pp. 2-3. (2) Green, S. W., The Quantitative Analysis o[ Mixtures o[ Chlorofiuoromethanes, in Desty, D. H., Vapor Phase Chromatography, Academic Press, New York, N.Y., 1957, pp. 388-94. (3) R•oot, M. J., and Maury, M. J., Gas chromatographic analysis of aerosol products, J. Soc. Cosmet. Chem., 8, 92-107 (1957). (4) Root, M. J., and Maury, M. ]., Volatile aerosol constituents, Soap Chem. Spec., 33, 101, 103, 105, 107 (April 1957). (5) Jenkins, J. w., and Amburgey, ]. M., Determination of volatile constituents of aerosols by gas chromat•ography, Proc. Sci. Sect. Toilet Goods Ass., 31, 19-21 (May 1959). (6) Jenkins, J. W., and Amburgey, J. M., Determination of volatile constituents of aerosols by gas chromatography, Aerosol Age, 4 (6), 35 (1959). (7) Brook, R. J., and Joyner, B. D., Analysis of aerosol propellants, J. Soc. Cosmet. Chem., 17, 401-14 (1966). (8) Cannizaro, R. D., Quantitative analysis of aerosol propellants by gas chromatography, Aerosol Technicomment Vol. XI, No. 1, Aerosol Techniques, Inc., Milford, Conn., Feb- ruary 1968. (9) Cannizaro, R. D., and Lewis, D. A., Gas chromatographic analysis of aerosols by pressur- ized liquid sampling, J. Soc. Cosmet. Chem., 20, 353-63 (1969). (10) Bourne, P. G., and Murphy, W. R., Identification and quantitative determination o[ propellants in aerosols, Ibid., 20, 525-37 (1969). (11) Cohen, Sheldon, Quantitative determination of volatile cotnponents in pressurized aero- sols by gas chromatography, J. Pharm. Sci., 57 (6), 966-70 (1958).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)