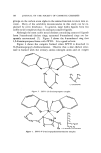
112 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS the succinic acid half-esters of fatty amides. The use of ingredients containing sodium ion should be avoided because of the possibility of hydrolizing the ZPTO at high pH's and forming the sodium pyrithione in situ. Other prototype hair preparations made (Table III) were a collagen- derived protein conditioner containing 0.2% solubilized ZPTO and a clear hair-thickening lotion based upon polyvinylpyrollidone that con- tained 0.5% solubilized ZPTO. Both of these preparations had a pH of 8.8. Related Amines Tested Besides the polyethylenimine, molecular weight 600, used in the previous formulations, it was found that polyethylenimines of higher molecular weights are also suitable. Polyethylenimines, for example, of molecular weights of 1,200, 1,800, and 60,000 were able to solubilize ZPTO in water. When the polyethylenimine function is ethoxylated or propoxylated, thus reducing the primary amine content, the solubilizing effect on ZPTO is reduced. If a modification of the polyethylenimine molecular structure is made so as to yield a linear polymeric molecule composed of only secondary amine functions, it has no solubilizing effect on ZPTO. It was found that when the molecular weight of polyethylenimine is reduced to g00, ZPTO is readily dissolved without the presence of water. Water can still be added to this solution without causing the precipitation of ZPTO. This was true of amines of even lower molecu- lar weight that may be considered to be derived from sections of the polyethylenimine molecule. Thus, tris(2-aminoethyl)amine and tetra- ethylene pentamine (Fig. 9) showed similar behavior to polyethylen- TRIS(2-AMINOETHYL)AMINE TETRAETHYLENE PENTAMINE Figure 9. Formulas for tris(2-aminoethyl)amine and tetraethylene pentamine
ZINC PYRITHIONE PREPARATIONS 113 imine of molecular weight 300. It is interesting that the building block of these •nolecules, ethylenediamine itself, does not dissolve ZPTO either with or without water. This is not unusual when one realizes that ethylenedia•nine is considered the weakest base among the polymethyl- enedia•nines, NH2(CH2)•NH2, due to its high inductive effect, its elec- trostatic repulsion effect, and intra•nolecular hydrogen bonding that should give the ethylenedia•nine •nolecule a •nost stable 54ne•nbered cyclic structure (13). CONCLUSION It was shown that clear hair preparations containing ZPTO can be prepared by first complexing the ZPTO in a variety of organic amines. Its solvation •nay be looked upon as an acid-base reaction in which the solubility of the ZPTO is a function of the amine cmnplexant. Among the factors that affect this solubility are the base strength of the amine, its steric hindrance, the formation of chelate rings, and its inductance and electrostatic effects, all of which contribute to the stability or in- stability of the complex. It is shown that if the proper precautions are taken, safe and effective clear preparations can be prepared. What should be determined is the minionurn concentration of complexed soluble ZPTO in such dear preparations that would be effective against dandruff. ACKNOWLEDGMENT The author would like to acknowledge and thank the following individuals who have contributed to the findings: Dr. Earle W. Brauer, Mr. Clyde M. Burnett, Mr. Bernard L. Kabacoff, and Mr. Radhakrishna B. Kasat. (Received May 28, 1971) REFERENCES (1) Brauer, E. W., Opdyke, D. L., and Burnett, C. M., Antiseborrheic qualities of zinc pyrithione in a cream vehicle, J. Invest. Dermatol., 47, 174 (1966). (2) Zinc and Sodium Compounds of Omadine, Tech. Bull., Olin Chemicals, New York, N.Y. (g) Opdyke, D. L., and Burnett, C. M., Private communication, Revlon Research Center, Aug. 25, 1965. (4) Baslo, F., and Johnson, R., Coordination Chemistry, W. A. Benjamin, Inc., New York, 1964, p. 126. (5) Yukawa, Y., Handboob of Organic Structural Analysis, W. A. Benjamin, Inc., New York, 1965, p. 584.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)